Dedicated to John Wolf
Viktor Reinhardt
Wisconsin Regional Primate Research Center,
University of Wisconsin, Madison
Received for publication September 14, 1992; revision accepted January 4, 1993.
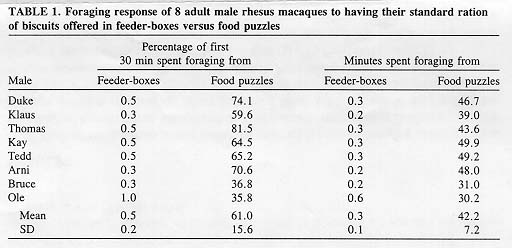
Ordinary feeder-boxes for macaques were converted into food puzzles by remounting them onto the square mesh (22 X 22 mm) of the front of the cages, away from original access holes. Feeding a standard ration of bar-shaped biscuits (40 X 24 X 16 mm; Purina Monkey Chow #5038; 236 g per animal), 8 adult pair-housed male rhesus macaques spent, on average, 61.0 ± 15.6% of the first 30 min retrieving biscuits from food puzzles, but only 0.5 ± 0.1 % from feeder-boxes. Their total amount of time engaged in gathering food was, on average, 141 times higher at food puzzles (42.2 ± 7.2 min) than at feeder-boxes (0.3 ± 0.1 min). It was concluded that using feeder-boxes as food puzzles, baited with the standard biscuit ration, offers a cost- and work-effective way to promote foraging activities in captive nonhuman primates.
Key words: macaques, feeding technique, foraging, environmental enrichment
INTRODUCTION
The promulgation of recent federal animal welfare rules [USDA, 1991] prompted numerous attempts to foster more foraging behavior in captive non-human primates. While animals in the wild invest substantial time in foraging [e.g., Teas et al., 1980; Marriott, 1988], animals in captivity are usually deprived of foraging opportunities; however, they will readily work for food even in the presence of freely available identical food [Anderson and Chamove, 1984].
The following foraging devices are available to comply with the new rules: mechanical operant devices [Markowitz, 1979], termite mounds [Nash, 1982], artificial gum-trees [McGrew et al., 1986], puzzle feeders [Bloomstrand et al., 1986; Line and Houghton, 1987; Bloom and Cook, 1989; Murchison, 1991], liquid dispensers [Bramblett and Bramblett, 1988; Crockett et al., 1988], pickup boards [Moazed and Wolff, 1988], foraging boxes [Meunier et al., 1989], pipe feeders [Bloomsmith, 1989; Maki et al., 1989], food dispensers [Markowitz and Line, 1989; Gullekson et al., 1991], suspended feeding stations [Molzen and French, 19891, puzzle boards [Chamove and Anderson, 1989; Brent and Eichberg, 1991], fleece boards [Bayne et al., 1992], fleece cushions [Lam et al., 1991], turf boards [Bayne et al., 1991 ], and food puzzles [Murchison and Nolte, 1992]. The highest stimulatory effect has been reported for turf boards, inducing adult rhesus macaques to spend on average 52% of a 30- min test session gathering and consuming flavored food particles [Bayne et al., 1992]. So far, foraging devices have not been designed as primary feeders; they are relatively expensive, and their maintenance is labor-intensive.
The present report presents results from an attempt to promote foraging behavior in caged non-human primates, using a more cost- and work-effective approach. Without any extra material, ordinary feeder-boxes were converted into food puzzles baited with the standard biscuit ration, instead of supplemental treats.
MATERIALS AND METHODS
Subjects of this study were eight healthy, 6-year-old male rhesus macaques (Macaca mulatta). They were housed as 4 compatible pairs, each pair in a 70 X 150 X 77 cm commercial lower-row double cage, equipped with a privacy panel, two perches and two gnawing sticks. Room temperature was maintained at 20-22oC, with a relative air humidity of approximately 50%, and a 12-hr light-dark cycle.
Water was available ad libitum. Each pair was fed a standard ration of 66 biscuits (476 g; Purina Monkey Chow #5038) once a day at 0900 hr, supplemented with fruit and bread or whole peanuts at about 1500 hr. The animals were accustomed to retrieving their food from two feeder-boxes, each 14 cm wide, 7 cm deep, 17 cm high, mounted 40 cm off the cage floor over a 73 X 47 mm access hole cut into the mesh on the front of each half of the cage.
The partners of each pair were observed simultaneously on two occasions:
(1) control situation on day 1: feeding of standard biscuit ration from the two feeder boxes; and
(2) experimental situation on day 30: feeding of standard biscuit ration from two food puzzles.
A food puzzle was created after completion of the control observation by remounting the feeder-box a few centimeters to the left or right of its original position, so that it no longer covered the access hole. (In some cage models, the bars or mesh of the door may be the only place suitable to accommodate the food puzzle.) To accomplish this work, 4-5 min were required. The new arrangement made it no longer possible for an animal to simply reach for food. Skillful manipulation with the fingers through the 22 X 22 mm square mesh was now required to retrieve the bar-shaped biscuits measuring about 40 X 24 X 16 mm (Fig. 1). The animals were habituated to using the food puzzles as primary feeders for I month prior to data collection on day 30.
Each pair was observed one time in both situations from 0900 hr until the last piece of biscuit was retrieved, i.e., up to 90.2 min for the control situation, and up to 102.4 min for the experimental situation. The following parameters were manually recorded with the help of stop-watches for each subject during the control and experimental situation:
1. Duration of foraging bouts (uninterrupted biscuit retrieval activity) expressed in seconds. Foraging bouts did not include periods of eating (masticating, filling/emptying cheek pouches and swallowing) that did not occur concurrently with food retrieval activities.
2. Percentage of first 1,800 s (30 min) spent foraging.
3. Total time (expressed in min) spent foraging.
Foraging was defined as food gathering activities [Random House Dictionary, 1987]. These included:
Foraging from feeder-boxes:
Collecting biscuits by simply reaching for them through the access holes
Foraging from food puzzles:
Maneuvering biscuits with the fingers into appropriate positions behind the mesh.
Pushing with the fingers and pulling with the fingers and/or incisors whole biscuits or parts of them through the mesh.
Breaking protruding parts of biscuits with the fingers or with the incisors.
Each animal was weighed on day 1, i.e., when the food puzzles were installed, and again on day 30, i.e., at the end of the habituation period. All animals were subjected to a dental check-up after having been exposed to food puzzles for 4 months. Statistical analysis was completed using the Student's t test and the Mann Whitney U test [Siegel, 1956].

RESULTS
Each of the 8 rhesus males showed a marked change in foraging activity, when the standard biscuit ration was placed in food puzzles, instead of in feeder-boxes. The average duration of foraging bouts increased from 1.3±0.9 sec to 48.7±35.2 sec (t = 3.623; P<0.001); the average percentage of time spent foraging during the first 30 min increased from 0.5% to 61.0% (U = 0; P<0.001); the average total time spent foraging increased from 0.3 min to 42.2 min (U = 0; P<0.001; Table 1).
Mean body weight balance of the animals was +2.5% (8.1±1.3 kg versus 8.3±1.2 kg) during the first month of food puzzle feeding, indicating no deficit in caloric intake, as a result of the increased foraging requirement. No special teeth wear or teeth damages could be detected in any of the 8 subjects, 4 months after the installation of the food puzzles.
DISCUSSION
Environmental enrichment for captive nonhuman primates does not need to be expensive. Creating one food puzzle by using already existing elements of the cage, i.e., ordinary feeder-box and mesh of cage front, required only a few minutes but no extra material. The effect of this simple change in cage design on rhesus macaques was surprising. Offering the standard biscuit ration in food puzzles instead of feeder boxes resulted in a 141-fold increase in time devoted to foraging.
The food puzzles not only served as primary feeders for biscuits, but they were also used to feed supplemental bread, whole peanuts and peeled bananas. Like biscuits, such food items require special manipulative skills from the animals to be retrieved from the food puzzles but not from the feeder-boxes.
The effectiveness of food puzzles in promoting foraging behavior prompted the conversion of feeder-boxes into primary food puzzles for more than 400 rhesus macaques and 43 stumptailed macaques at the Wisconsin Primate Research Center.
CONCLUSIONS
The present study is based on a relatively small subject pool. Its findings are preliminary but so conspicuous that they justify the following conclusions:
1. Converting feeder-boxes into food puzzles is a low-cost environmental enrichment project
2. Requiring no special maintenance and being used as primary feeders, food puzzles are particularly labor-inexpensive.
3. Food puzzles foster foraging behavior, thereby counteracting understimulation.
4. Working for their standard biscuit ration, by skillfully retrieving it from the food puzzles instead of simply removing it from feeder-boxes, has no immediate adverse impact on the animals' general health, as reflected in body weight development.
5. It is likely that food puzzles can be used as effectively by other nonhuman primates as well.
6. Food puzzles may not be suitable for animals with worn or unhealthy incisors.
ACKNOWLEDGMENTS
The manuscript benefited from very constructive comments by three anonymous reviewers and my wife Annie. I am thankful to Jackie Kinney for proofreading the text. This project was supported by NIH grant RR-00167 to the Wisconsin Regional Primate Research Center, publication No. 32-026 WRPRC.
REFERENCES
Anderson, J.; Chamove, A.S. Allowing captive primates to forage. Pp. 253-256 in STANDARDS IN LABORATORY ANIMAL MANAGEMENT, VOL. 2. Potters Bar, UK, Universities Federation for Animal Welfare, 1984.
Bayne, K.; Mainzer, H.; Dexter, S.; Campbell, G.; Yamada, F.; Suomi, S. The reduction of abnormal behavior in individually housed rhesus monkeys (Macaca mulatta) with a foraging/ grooming board. AMERICAN JOURNAL OF PRIMATOLOGY 23:23-35, 1991.
Bayne, K.; Dexter, S.; Mainzer, H.; McCully, C.; Campbell, G.; Yamada, F. The use of artificial turf as a foraging substrate for individually housed rhesus monkeys (Macaca mulatta). ANIMAL WELFARE 1:39-53, 1992.
Bloom, K.R.; Cook, M. Environmental enrichment: Behavioral responses of rhesus to puzzle feeders. LAB ANIMAL 18(5):25-30, 1989.
Bloomsmith, M.A. Feeding enrichment for captive great apes. Pp. 336-356 in HOUSING, CARE AND PSYCHOLOGICAL WELL-BEING OF CAPTIVE AND LABORATORY PRIMATES. E.F. Segal, ed. Park Ridge, New Jersey, Noyes Publications, 1989.
Bloornstrand, M.; Riddle, K.; Alford, P.; Maple, T. Objective evaluation of a behavioral enrichment device for captive chimpanzees (Pan troglodytes). ZOO BIOLOGY 5:292- 300, 1986.
Bramblett, R.D.; Bramblett, C.A. A liquid dispenser for caged primates. LABORATORY PRIMATE NEWSLETTER 27(4):16, 1988.
Brent, L.; Eichberg, J.W. Primate puzzleboard: A simple environmental enrichment device for captive chimpanzees. ZOO BIOLOGY 10:353-360, 1991.
Chamove, A.S.; Anderson, J.R. Examining environmental enrichment. Pp. 183-202 in HOUSING, CARE AND PSYCHOLOGICAL WELL-BEING OF CAPTIVE AND LABORATORY PRIMATES. E.F. Segal, ed. Park Ridge, New Jersey, Noyes Publications, 1989.
Crockett, C.; Bielitzki, J.; Carey, A.; Velez, A. Kong toys as enrichment devices for singly caged macaques. LABORATORY PRIMATE NEWSLETTER 28(2):21-22, 1988.
Gullekson, R.; Bench, L.; Harrigan, K.; Pyle, K. Seed-feeder as a foraging device for singly housed cynomolgus monkeys (Macaca jascicularis). LABORATORY ANIMAL 20(6):44- 46, 1991.
Lam, K.; Rupniak, N.M.J.; Jversen, S.D. Use of a grooming and foraging substrate to reduce stereotypies in macaques. JOURNAL OF MEDICAL PRIMATOLOGY 20:104 - 109, 1991.
Line, S.W.; Houghton, P. Influence of an environmental enrichment device on general behavior and appetite in rhesus macaques. LABORATORY ANIMAL SCIENCE 37:508, 1987.
Maki, S.; Alford, P.L.; Bloomsmith, M.A.; Franklin, J. Food puzzle device simulating termite fishing for captive chimpanzees (Pan troglodytes). AMERICAN JOURNAL OF PRIMATOLOGY SUPPLEMENT 1:71-78, 1989.
Markowitz, H. Environmental enrichment and behavior engineering for captive primates. Pp. 217-238 in CAPTIVITY AND BEHAVIOR: PRIMATES IN BREEDING COLONIES, LABORATORIES AND ZOOS. J. Erwin; T.L. Maple; G. Mitchell, eds. New York, Van Nostrand Reinhold, 1979.
Markowitz, H.; Line, S. Primate research models and environmental enrichment. Pp. 202-212 in HOUSING, CARE AND PSYCHOLOGICAL WELL-BEING OF CAPTIVE AND LABORATORY PRIMATES. E.F. Segal, ed. Park Ridge, New Jersey, Noyes Publications, 1989.
Marriott, M.B. Time budgets of rhesus monkeys (Macaca mulatta) in a forest habitat in Nepal and on Cayo Santiago. Pp. 125-149 in BEHAVIOR OF FOOD-ENHANCED PRIMATE GROUPS. C.H. Southwick; J.E. Fa, eds. New York, Alan R. Liss, 1988.
McGrew, W.C.; Brennan, J.A.; Russell, J. An artificial "gum-tree" for marmosets (Callithrix j. jacchus). ZOO BIOLOGY 5:45-50, 1986.
Meunier, L.D.; Duktig, J.T.; Landi, M.S. Modification of stereotypic behavior in rhesus monkeys using videotapes, puzzle feeders, and foraging boxes. LABORATORY ANIMAL SCIENCE 39:479, 1989.
Moazed, T.C.; Wolff, A.V. The raisin board as an environmental enrichment tool for laboratory primates. LABORATORY PRIMATE NEWSLETTER 27(l):16, 1988.
Molzen, E.M.; French, J.A. The problem of foraging in captive callitrichid primates: Behavioral time budgets and foraging skills. Pp. 89-101 in HOUSING, CARE AND PSYCHOLOGICAL WELL-BEING OF CAPTIVE AND LABORATORY PRIMATES. E.F. Segal, ed. Park Ridge, New Jersey, Noyes Publications, 1989.
Murchison, M.A. PVC-pipe food puzzle for singly caged primates. LABORATORY PRIMATE NEWSLETTER 30(3):12-14, 1991.
Murchison, M.A.; Nolte, R.E. Food puzzle for singly caged primates. AMERICAN JOURNAL OF PRIMATOLOGY 27:285-292, 1992.
Nash, V.J. Tool use by captive chimpanzees at an artificial termite mound. ZOO BIOLOGY 1:211-221, 1982.
Random House Dictionary. New York, Random House, 1987.
Siegel, S. NONPARAMETRIC STATISTICS FOR THE BEHAVIORAL SCIENCES. New York, McGraw-Hill, 1956.
Teas, H.; Richie, T.; Taylor, H.; Southwick, C. Population patterns and behavioral ecology of rhesus monkeys (Macaca mulatta) in Nepal. Pp. 247-262 in THE MACAQUES: STUDIES IN ECOLOGY, BEHAVIOR AND EVOLUTION. D.G. Lindburg, ed. New York, Van Nostrand Reinhold, 1980.
U.S. Department of Agriculture. Animal Welfare, Standards, Final Rule. FEDERAL REGISTER 56:6426-6505, 1991.
This article originally appeared in Zoo Biology 12, 307-312 (1993).
Reprinted with permission of the Editor.