Hilary O. Box and Brigitte Rohrhuber
Department of Psychology,
University of Reading, England, UK.
German Primate Center, Göttingen, FRG.
INTRODUCTION
Recent interest in the welfare of captive animals has led to the use of techniques to stimulate normal patterns of behaviour and maintain healthy, reproductively viable individuals.18,19 Techniques include naturalistic methods such as foraging and clearly artificial ones, as with a variety of problem solving devices.10 Such techniques are relatively high profile in that they may create an 'immediate' change in the time budgets of behavioural activities in a more 'favourable' direction, i.e. individuals spend less time engaged in activities that are considered to be counter-productive to their welfare.15 Many laboratory animals however, do not live in environments that are specifically designed to enrich their behaviour. In fact, experimental protocols frequently require forms of standardised and uniform environments. In such cases the cages used are often determined by commercial constraints and folklore among institutions. Moreover, National and International guidelines of the dimensions of cage sizes and furnishings for different species are based, for example, upon body weight and postural propensities and not upon empirical comparisons of behavioural and/or physiological responsiveness among animals in commonly used conditions of laboratory housing. This is an omission both on welfare and scientific grounds. We advocate that one critical aspect of welfare studies should be to compare directly the behaviour of the same species of animal in different, commonly occurring laboratory conditions, including variations in temperature, humidity, light, cage size and furnishings. Cage size, for example, is an especially contentious issue and, in the absence of comparative research, people tend to assume that increases in space for the same social density and composition will be automatically beneficial in stimulating activities such as locomotion and exploration. In fact, studies with different species of callitrichid for instance, have shown that this is not necessarily so.3,13 Moreover, studies with various primate taxa show that the issues are complex, and deserve close scrutiny for different species.2
The present report concerns the cotton top tamarin (Saguinus oedipus oedipus). This species is of interest on welfare grounds due to its endangered and highly precarious position in nature, its frequent use in biomedical research, and the fact that it does not do well under a variety of 'standard' laboratory maintenance. Indeed, attention has been drawn to high incidences of infant mortality and abortion; in short, to poor breeding success.11 Among those few establishments that have maintained healthy viable breeding colonies of cotton tops, it has been observed that association with large complex and changing environments in which there is variety and frequent social and physical stimulation is of benefit.20
The aim of the present study was to compare the behaviour of pairs of tamarins in conditions that are more frequently found in laboratories, and in which for economic and other reasons, specific programmes of enrichment and variety are not provided. It was of particular interest to compare the behaviour of tamarins in a commercial custom built rack system with that which provided considerably more space and furniture with reference to equivalent social density. Further, single adult male female pairs of tamarins were selected because this is the breeding unit that is set up for callitrichid species in captivity.
METHODS AND MATERIALS
A total of seventeen adult male, female pairs of cotton tops were observed. Their ages ranged from 3 to 9 years. With the exception of one of the pairs, none had bred successfully.
Condition I
Eight pairs were selected at random from a total of sixteen pairs, housed together in one room that contained two horizontal rows of eight cages, with one row independently racked above the other. Each cage measured 1.0 m wide x 0.7 m high and 0.5 m deep. It was equally divided vertically with a wooden dowel as a perch along the length of the cage. [FIGURE I].
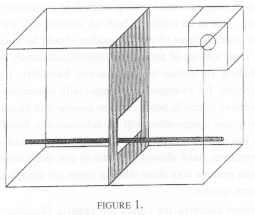
Two observers simultaneously scanned the behaviour of separate pairs of animals from within a hide which made them invisible to the monkeys. Observations were made between 11 a.m. and 3 p.m. on three days per week (Monday, Wednesday, Friday) over a period of seven consecutive weeks. Each pair was observed for 10 min per session with the prearranged order in which the pairs were watched, randomised over days. A maximum of four, 10 min observations was made on anyone group of tamarins on anyone day. A total of 2,400 ten-second time samples of behaviour was recorded for each animal in this condition.
Condition 2
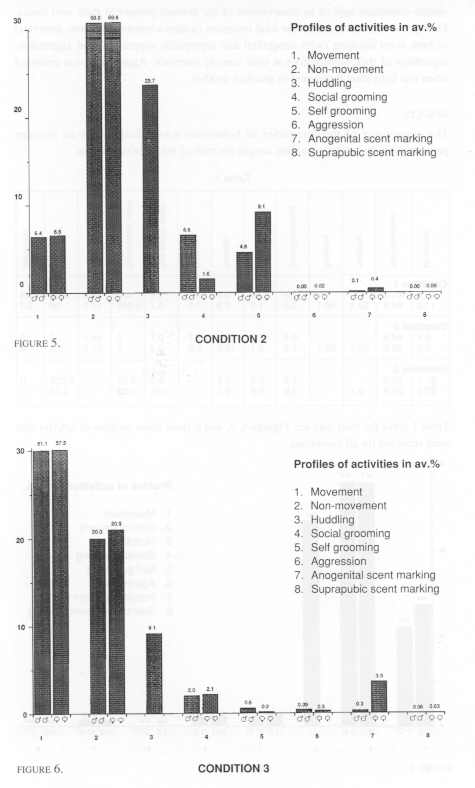
Three pairs of cotton tops were included. Two of these pairs lived in one room with no other tamarins present. The third pair was one of two pairs living in a different, but identically arranged room. All these monkeys lived in wooden frame and wire mesh cages of dimensions 1.7 m high x 0.75 m deep and 0.9 m wide. The cage furniture consisted of a nest box (as in Condition 1, but made of wood), two triangular platforms, and three branches - one horizontally across the cage, and two vertically across the cage at 45º [Figure 2].
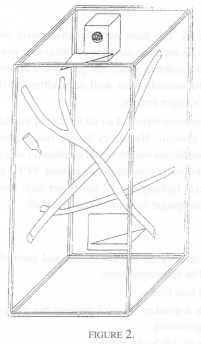
As before, observations were made on the behaviour of each pair simultaneously - from a hide, 2,400 ten-second time samples of behaviour were recorded per animal, this time over 5 days per week during a five consecutive week period, with the order of pairs again randomised.
Condition 3
Six pairs of cotton tops were distributed among three rooms with each of the two pairs per room living in a cage that measured 1.6 m x 2.4 m x 2.0 m. Branches were placed horizontally across the room at two levels, and a wooden nest box was also provided as in Condition 2 (Figure 3).
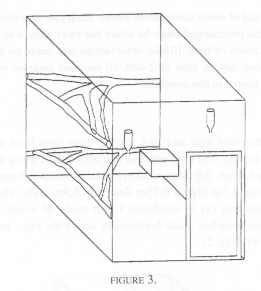
The pairs were simultaneously observed by one of us (BR) for a total of 3000 thirty-second time samples of behaviour over a period of twenty five weeks. Once again, the order in which the pairs were observed was randomised. There was no hide but the animals were well accustomed to the observer as these observations were part of a larger project.
In all conditions, the tamarins were fed an ad lib diet of monkey chow, fresh fruits, vegetables and animal protein. Humidity levels ranged around 55-60%. The temperature in the three conditions varied in that Condition 1 was maintained at around 76°F (±2) with that in the other conditions at around 74°F (±2). All the keeping rooms were lit by artificial lighting for 12 hours per day. Rooms in the first two conditions also had a single opaque window in one end wall.
Categories of behaviour
Behaviour was recorded directly on to data sheets, and cued electronically into ear pieces for the observers. The categories were:
- Movement - involving all four limbs.
- No movement - in which a monkey was not moving but did not engage in another recorded activity such as grooming.
- Huddling - stationary side to side body contact.
- Autogrooming - manipulation of an animal's own pelage.
- Social grooming - close visual inspection and manipulation of the pelage of another animal.
- Feeding - ingestion of food items.
- Drinking - taking water from water bottle.
- Stereotyped behaviour - repetitive atypical behaviour of the normal repertoire for the species.
In addition, recordings of proximity, i.e. when individuals were within 30 cm of one another (for whatever activity except moving) was observed in Conditions 1 and 2. A similar constraint applied to observations of the animals present in their next boxes. Finally, we decided to record the total instances (within a number of 10 sec. intervals) of both scent marking (with anogenital and suprapubic separately) and aggression, regardless of their occurrences at time sample intervals. Aggression was recorded when one individual threatened or attacked another.
RESULTS
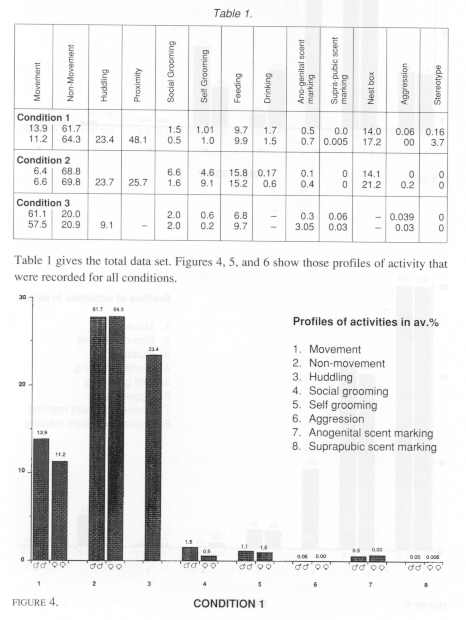
The occurrence of the categories of behaviour was calculated as an average percentage of the total observation sample for each of the three conditions.
Statistical comparisons were made initially on these common categories. Because no difference in behaviour was found among males and females, that is no correlation (Spearmans, rho) was found within male/female pairs for any condition, comparisons of all categories of behaviour were made by Mann-Whitney U test (two tailed). The only significant difference was that females scent marked anogenitally more than males in Conditions 2 and 3 (U = 3.5, df=8, p<0.05). No such difference was found in Condition 1.
A central interest was to test for significant differences among common behavioural categories in the relatively small, 'medium' and large cages; comparisons were made by using a Kruskal-Wallace test. Two significant differences were found. The tamarins in Condition 3 (the largest) were significantly more active in terms of gross locomotor activity (movement category) than those in and between Conditions 1 and 2 (k=1694.6, k=166.3, p<.01). There was also significantly less close physical contact (huddling category) within pairs in condition 3 than those in and between Conditions 1 and 2. It was of particular interest however, to find that stereotyped behaviour (head bobbing) was only observed in Condition 1 and among females especially. We may note that not all the females exhibited the behaviour, but that it was a prevalent activity that we have never observed under any other laboratory condition. Subsequently, it was of interest to examine whether there were significant correlations between the behaviour of tamarins which lived in the same rooms. In fact, there were not. Moreover, the arrangement of condition 1 gave an opportunity to compare the behaviour of animals caged in different positions in the room (Mann-Whitney). The eight groups that we observed were labeled from left to right in each case, A,B, 1 and 2 on the top rack, and C, D, 3 and 4 on the lower rack. We found that those tamarins that were housed on the left hand (window) side of the room showed significantly more gross locomotor behaviour than those on the right hand side. Groups A+C vs 2+4 (df=4, U=0.00, p<0.05) - groups A+C+B+D vs 1+2+3+4 (df=8, U=5.0, p<0.01).
Again, those tamarin that were housed in the top bank of cages 1+2+A+B showed significantly more close physical contact (huddling) than those housed on the bottom bank 3+4+C+D (df= 8, U=8, p<0.01). They also showed significantly less behaviour recorded as 'non-movement' than those housed on the bottom bank (df=8, U=1, p<0.01). No other significant differences in any other category of behaviour was found in either of these regards.
Finally, we may comment briefly on differences in those categories of behaviour that were recorded in Conditions 1 and 2 only. For example, despite considerable differences in the space available between these conditions, the tamarins spent similar amounts of the observation time in their nest boxes. The males were very similar in this regard with the females in each case spending more time than their pair mates.
We also recorded more drinking in Condition 1. Given that this applied to both males and females, the differences are likely to be attributable to differences in the amount of water available in the diet, and for the higher temperature in Condition 1, than as suggesting reproductive condition (as early pregnancy) in the females, for instance.
Further, our measures of proximity within pairs of tamarins in Condition 1 were nearly twice those of Condition 2. This may be hardly surprising given the overall size differences of the cages. However, these differences do not necessarily indicate any measure of social integration. Differences between the two conditions in close contact scores, for example, were not significant.
DISCUSSION
Our results show significant differences in the behaviour of pairs of cotton top tamarins maintained in different conditions of housing. One such difference, and one that might not have been intuitively suggested, relates to the relative position of cages in a single keeping room (Condition 1). Hence, those pairs of tamarins that lived in cages in the half of the room nearer to the one opaque window, including both top and bottom racks in this half, were more active (gross ambulation) than those housed on the other side. Further, the animals that lived in the top rack of cages in the whole room engaged in more close contact amicable behaviour of huddling, and less behaviour recorded as inactive, than those on the bottom rack.
There are few reports from which hypotheses are suggested to account for such findings, but one variable that is at least partially relevant concerns the relative amounts of light available in the room. Hence, although we did not actually measure levels of illumination in the different areas, there are similar findings to our own, albeit in another callitrichid of a different genus. The results are worthy of discussion for their implications for laboratory management. Hence, Heger and Neubert (1988) describe the maintenance of a colony of common marmosets (Callithrix jacchus) in which male, female pairs lived in cages 70 x 70 x 50 cm. Some pairs lived in rooms that were darker than the original colony room in which marmosets continued to live.
Breeding records showed a lower breeding rate for these marmosets, than for those in the lighter, original room. Moreover, because records were taken over several years, it is reasonable to discount the influence of disturbance by moving the animals. Further, subsequent manipulation of relative illumination levels confirmed the novel finding.8Again, differences in fertility were also found between animals living in upper racked (lighter) cages than in lower ones. Most important, breeding success (pregnancy rate and litter size) could be reliably increased by manipulating light levels. We have no records of the long term breeding success on the cotton tops in our Condition 1, but they were not breeding successfully at the time of our observations. In our study, different levels of illumination were linked to significantly different levels of locomotion. This is similar to Scott's (1991) finding for a system in her laboratory in which single common marmosets, housed in two tier racks in cages 75 cm high x 50 cm wide x 60 cm deep showed significantly less locomotor activity in the lower tier than in the higher, lighter one. It is difficult to know whether the animals in lighter conditions gained any advantage in terms of 'well being'. Certainly activity is important to prevent joint stiffness for example, but it is not necessarily the case that a lower level of activity has deleterious effects. It is critical, however, that light per se, can clearly be influential in stimulating behavioural and reproductive activity. There is also information to suggest interesting and therapeutic influences of light and visual stimulation in ways that are, as yet, inextricably mixed.
Some studies have shown an advantageous influence of access to windows upon health and mood in humans21,17 which in this context one notes that rotating monkeys into lighter, more visually stimulating areas in a keeping room may present a valuable source of environmental enrichment.
Another interesting aspect of the results from the present study concerns the fact that observers frequently encounter the concern that the behaviour of particular animals or social groups are indeterminately influenced by the behaviour of the animals in the same immediate environment; that such influences may turn out to be uncontrolled variables in many studies. It was of interest to test for this effect in our study, and to find that there was no such pervasive influence. It was also of interest to examine the occurrence of gender differences in behaviour. In fact, the only significant difference here, and one that is in line with many reports in the literature;2 is that females scent marked more anogenitally than males in both Conditions 2 and 3, but not in Condition 1. It is unusual to find a captive situation in which females living with males do not scent mark more. The reasons why this should not have been the case in Condition 1 remain obscure.
A central point of concern of our study related to the potential influence of space per se. The cages of the three different conditions of housing varied considerably in overall space; they also varied in the quantity of cage furniture available. However, none of them was purposefully 'enriched' and represented types of cages which are routinely found in different laboratories. We might expect, as indeed we found, that tamarins living in Condition 3, in which the space was considerably larger than in either of the other two conditions, moved around more. On the other hand, it is worth noting that the present arrangement of our common marmoset colony at Reading involves family groups living in large cages 2 m wide by 4 m long by 3 m high. Here we find that adult pairs (and especially males) move around relatively little; they move around less than before in smaller cages and they tend to maintain locational 'stations' spatially independent of all other individuals for much of the time (Box and Smith, unpublished data). In any case, it is important to point out that the interface between space and social opportunities to express patterns of social interactions among individuals of different age and gender is complex and a largely unexplored area in animal welfare. However, in the present study, no significant differences in locomotor activity was found between Conditions 1 and 2. This may be regarded as an unexpected finding given that the two conditions varied considerably in available space. All this assumes that a major advantage of additional space is to promote locomotor activity. It is also important,of course, to examine the quality of interactions within social units of individuals. Two points are salient here. One is that there was no significant difference in close amicable behaviour as evidenced by huddling at least, between the smaller commercial unit and Condition 2. In fact, the significant difference in this regard was that close contact was less frequent in the much larger Condition 3, where the animals moved around more. If we take into account that there was no significant difference among the tamarins in their social (and autogrooming) activities, and that neither was there any difference in their overt aggressive behaviour (which was very low anyway) then there were no 'immediate' social behavioural indicators of response to environmental 'stressors.' On the other hand, it is significant that we observed a stereotypic head bobbing behaviour in Condition 1 only, and that this was far more prevalent among females than males. Quite why there should be such a marked gender difference in this respect is unknown, but it is certainly worthy of further consideration from theoretical (stress responsiveness) and practical (animal welfare and management) points of view. Moreover, ' small cage' size has long been associated with stereotyped movements in a wide variety of species6 and there are numerous examples with primates.4,16,5,2 There does remain, however, the question of the extent to which stereotypic movements indicate poor animal welfare. There are those who argue in favour of such a view1 and others12 who claim that the argument remains open. Certainly, in our study, we had no clearly unambiguous indicators of poor welfare such as self mutilation. Moreover, if we allow the ambiguity of the reference of stereotyped behaviour, then there are no obvious and immediate behavioural grounds for choosing among the three different conditions of housing that we used in this study. On the other hand, the absence of clearly deleterious behaviour does not necessarily lead us to advocate the environments we have described. It is our belief that on both scientific and welfare grounds, these animals are much better kept in enriched conditions that take more account of their species propensities and characteristics, and especially if we wish to promote successful breeding. However, at this stage, we must also allow that although there is much assumption about the deleterious and advantageous influences of different types of laboratory caging for the health and welfare of simian primates, empirical comparisons are rare.5 Moreover, studies of the influence of environmental variables, such as cage size, require careful methodological consideration. For example, one technique for comparing the influence of specific cage conditions is to record behaviour in one area, and then move the animals to the other area. In these cases, the possibility that individuals are initially responding to novel features of the 'new' environmenr4 should be allowed for by sufficiently long term observations to reach acclimatisation. Another method of course, is to adopt a cross sectional approach in which the equivalent social density and structure of particular species are compared in different degrees of the spatial density in which they are normally kept. Both approaches are potentially useful and complementary in different contexts. We adopted a cross sectional approach in this study in order to answer some practical questions. Perhaps the most important point to emphasise at this preliminary stage is that sets of identically designed cages in a system that looks very simple, with minimum expense and maintenance, that are arranged economically with regard to the number of animals per unit space may not, in fact, provide 'standard' laboratory conditions. The implications of such findings should be recognised by those working in laboratory animal science.
Acknowledgements
B.R. wishes to thank the British Council for their financial support of her work at Reading University. Special thanks are due to Adam Terlecki for all his help and friendliness during the observations in Condition 1.
References
1. Broom, D.M. (1988) Sow welfare indicators. Veterinary Record, 23 (9): 235.
2. Bayne, K.A.L. and McCully, C. (1989) The effect of cage size on the behaviour of individually housed Rhesus monkeys. Lab. Animal (October issue): 25-28.
3. Chamove, A.S. and Rohrhuber, B. (1989) Moving Callitrichid monkeys from cages to outside areas. Zoo Biology 8: 151-163.
4. Draper, W.A. and Bemstein, I.S. (1963) Stereotyped behaviour and cage size. Perceptual and Motor Skills, 16: 231-234.
5. Harris, D. (1988) Welfare and housing of old-world non-human primates. UFAW Animal Welfare Research Report No.1. Potters Bar: UFAW .
6. Hediger, H. (1968) The Psychology and Behaviour of Animals in Zoos and Circuses. Dover. New York.
7. Heger, W. and Neubert, D. (1988) Maintenance and breeding of Callithrix jacchus in a colony in Berlin. In: D. Neubert; H.J. Merker; A.G. Hendricks (Eds) Non-human primates - Developmental Biology and Toxicology. Verlag. Berlin.
8. Heger, W., Merker, H.J., Neubert, D. (1986) Low light intensity decreases the fertility of Callithrix jacchus. Primate Report, 14, : 260.
9. McGrew, W.C., Brennan, J.A. and Russell, J. (1986) An artificial "Gum-tree" for marmosets (Callithrixj.jacchus). Zoo Biology, 5: 45-50.
10. Markowitz, H. (1982) Behavioural Enrichment in the Zoo. Van Hostrand Rheinhold. New York.
11. Kirkwood, J .K., Epstein, M.A. and Terlecki, A.J. ( 1983) Factors influencing population growth of a colony of cotton-top tamarins. Laboratory Animals, 17: 35-41.
12. Line, S.W. (1987) Environmental enrichment for laboratory primates. Journal of the Veterinary Medical Association. 190 (7): 854-859.
13. Mager, W.B. and Griede, T. (1986) Using outside areas for tropical primates in the Northern Hemisphere: Callitrichidae, Saimiri, and Gorilla. In: K. Benirschke (Ed) Primates -The Road to Self-Sustaining Populations. Springer-Verlag, pp.471-477.
14. Nash, L.T. and Chilton, S. (1986) Space or novelty?: Effects of altered cage size on galago behaviour. American Journal of Primatology 10: 37-49.
15. Novak, M.A. and Drewson, K.H. (1989) Enriching the lives of captive primates: issues and problems. In: E.F. Segal (Ed.) Housing, Care and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications. New Jersey. pp.161-182.
16. Paulk, H., Dienske, H. and Ribbens, L. (1977) Abnormal behaviour in relation to cage size in rhesus monkeys. Journal of Abnormal Psychology, 86: 87-92.
17. O'Neill, P. (1989) A room with a view for captive primates: issues, goals, related research and strategies. In: E.F. Segal (Ed) Housing, Care and Psychological Wellbeing of Captive and Laboratory Primates. Noyes publications. New Jersey, pp.35-160.
18. Poole, T.B. (1991). Criteria for the provision of captive environments. In: H.O. Box (Ed.) Primate Responses to Environmental Change. Chapman and Hall. London. p.357-374.
19. Scott, L. (1991) Environmental enrichment for single housed common marmosets. In: H.O. Box (Ed.) Primate Responses to Environmental Change. Chapman and Hall. London. pp.265-274.
20. Snowdon, C. T., Savage, A. and McConnell, P. B. (1985) A breeding colony of cotton-top tamarins (Saguinus oedipus). Laboratory Animal Science, 35 (5): 477-480.
21. Ulrich, H. J. (1978) Some aspects of role talking behaviour in captive family groups of the cotton top tamarin (Saguinus oedipus oedipus). In: Rothe, H., Wolters, H. J., Ream, J. P. Biology and behaviour of marmosets. Eigerneslag Rothe, Göttingen. pp.259-280.
Reproduced with permission of the Institute of Animal Technology.