S J Schapiro and D Bushong
The University of Texas M D Anderson Cancer Center, Science Park,
Department of Veterinary Sciences, Route 2, Box 151-B1, Bastrop, Texas 78602, USA
Abstract
For captive primates, environmental enrichment may improve psychological well-being, as indicated by changes in the frequency of species-typical and abnormal behaviours. The effects of enrichment on physical well-being have also been examined, but little attention has been devoted to the relationship between enrichment and animal health. We therefore studied the health records of 98 rhesus macaques (Macaca mulatta) to measure the effects that enrichment and social housing manipulations had on the number of veterinary treatments and days of therapy required by the monkeys. Subjects were housed singly, in pairs and in groups. Half of the subjects in each housing condition were enriched and the others were controls. Control and enriched subjects did not differ in the number of treatments they required, but enriched subjects received longer therapies than did controls. Neither treatments nor days of therapy were frequent or randomly attributed across housing conditions, pair-housed subjects required the least treatment and therapy, whereas singly housed subjects were treated slightly more frequently for diarrhoea -related problems, and group-housed subjects for trauma-related problems. Subject age, however, was a potential confounding factor. Because subjects were part of a specific pathogen-free breeding programme, they spent only certain ages in each housing condition. Results suggest that inanimate enrichment neither diminishes nor improves the health of young macaques, but that enriched monkeys may require longer periods of therapy than do controls. Pair housing may be an effective housing strategy from both veterinary and behavioural points of view, necessitating relatively few treatments, but providing some social enrichment opportunities.
Keywords: animal welfare, environmental enrichment, pair housing, physical well-being, psychological well-being, rhesus macaques
Introduction
Current criteria for defining psychological well-being in captive primates include references to physical, physiological and behavioural indices (Novak & Suomi 1988, Mason 1991). Using these criteria, many environmental enrichment techniques have been shown to be beneficial for captive primates, including physical (Bryant et al 1988, Schapiro & Bloomsmith in press); feeding (Bloomsmith et al 1988, Molzen & French 1989, Schapiro & Bloomsmith in press); occupational (Chamove et al 1982, Bayne et al 1991), and sensory (Brent et al 1989; Bloomsmith et al 1990) enhancements to the captive setting. In most instances, the benefits of enrichment have been measured by behavioural changes; more specifically, increases in the frequency of species-typical behaviours are taken as indication of an improvement in psychological well-being (Novak & Suomi 1988; Line & Morgan 1991). Studies have also examined the effects of environmental enrichment on physical well-being of captive primates, examining physiological variables such as heart (Line et al 1989), body-weight (Clarke et al 1989; Bayne et al 1991; Schapiro & Kessel 1993), reproductive parameters (Eaton et al 1992) and cortisol levels (Champoux et al 1989; Novak & Drewsen 1989; Byrne & Suomi 1991; Crockett et al 1991; Schapiro et al 1993a)... However, only a few studies have scrutinized the effects of inanimate manipulations which might be considered as enrichment on the incidence of specific health problems (Erwin 1977; Erwin & Sackett 1990). If inanimate enrichment procedures enhance psychological well-being by reducing stress and promoting species-typical behaviour patterns, then physical well-being would be expected to improve as well, with a concomitant decrease in the number of severity of health problems.
The opportunity to interact with conspecifics, a social enhancement compared with sit housing, has been shown to enhance psychological well-being (Reinhardt et al 1987, 1988; O'Neill et al 1991; Schapiro et al 1993b; Schapiro & Bloomsmith in press [a], in press submitted; but see Ruppenthal et al 1991). When possible, pairing instead of single-housing, is currently recommended, because the expression of species-typical behaviour, an important criterion for determining psychological well-being (Novak & Suomi 1988), is more likely as the social situation approaches the species norm. Health issues associated with social manipulations (whether for enrichment purposes or not) have been studied (Eaton et al 1991), emphasizing the potential for trauma-related injuries when animals are initially introduced (Bernstein et al 1974; Erwin 1977; Bernstein 1991; Crockett et al 1991). If social enrichment procedures can improve psychological well-being by providing appropriate outlets for species-typical activities, then a corresponding improvement in physical well-being is expected. Following this logic then, the frequency and severity of health problems among socially-housed primates would be expected to be lower than for singly-housed subjects.
One must also be concerned with the possible negative consequences of enrichment on physical well- being of captive primates. Enrichment foods or bedding may provide additional( opportunities for digestive, nutritional, or infectious disease problems and manipulable, objects, perches, toys etc. may provide additional opportunities for injury. Most type; inanimate enrichment increase the difficulty and quantity of required husbandry procedures. Inadequate completion of newly complicated husbandry tasks may increase the likelihood of unsanitary conditions (Bayne et al 1993), which may in turn increase the likelihood of he problems. Therefore, the veterinary requirements for primates that receive these type, inanimate enrichment might be expected to be higher as a function of having to treat disorders or injuries associated with enrichment. Clearly, housing primates socially may increase their veterinary requirements, particularly in relation to infectious disease and previously mentioned trauma during group formation. The present study was undertaken to determine whether environmental enrichment affects the physical well-being of captive rhesus macaques living alone, in pairs and in small breeding groups, as measured by the amount of veterinary care they required.
A traditional rhesus macaque (Macaca mulatta) breeding colony is in the process of being converted into a specific pathogen-free (SPF) colony at The University of Texas M D Anderson Cancer Center, Science Park (Voss et al 1991). The goal is to rid the colony of four viruses (Herpesvirus simiae, Simian Immunodeficiency Virus, Simian Retrovirus and Simian T-cell Lymphotropic Virus) using a derivation process involving several manipulations in housing and social grouping. Subjects live for their first year in the natal social group and are then relocated to single cages for their second year. The monkeys' third year is spent paired with an opposite- sex partner of similar age and rearing history. In the last phase of the derivation programme, during the fourth and subsequent years, breeding groups of one male and four to seven females as well as all-male groups are created. This housing programme was designed to minimize viral transmission and behavioural disturbance by accounting for crucial periods of viral and behavioural development.
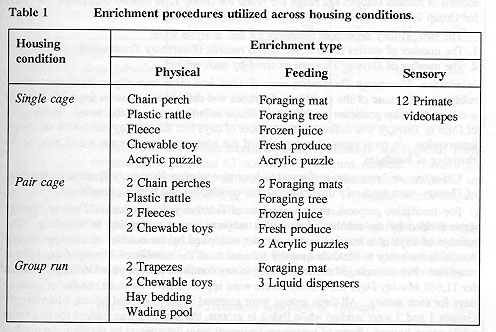
Throughout this programme, groups of enriched subjects (those which received several types of environmental enrichment) in addition to groups of control subjects (those which did not receive any enrichment) were maintained. During the single caging phase of the derivation, enriched subjects were presented with physical enrichment for three months, feeding enrichment for six months and sensory enrichment for the remaining three months (see Table 1 and Schapiro et al 1991; Schapiro & Bloomsmith 1994 & 1995, for details of enrichment). Throughout the pair and group housing phases of the programme, enriched subjects simultaneously received a combination of physical and feeding enhancements. The control groups provide ideal comparison groups for examinations of the health and veterinary care requirements of enriched groups. Since animals were maintained in three different housing conditions, comparisons can also be made that examine housing effects, as well as any interactions between enrichment and housing condition.
Methods
The medical records of 98 subjects were reviewed. These animals came from three birth cohorts; one born in 1988 (Group 1), one born in 1989 (Group 2) and one born in 1990 (Group 3). In each cohort, half of the animals were enriched and the other half were control subjects. Health record entries for illness or treatment were tabulated beginning at one year of age when the animals were separated from the natal group and placed in single cages. Data for Groups 1 and 2 came from all three housing conditions, while the data for Group 3 were from single and pair housing only. Data were analysed from approximately 48 months of records (subject age range 1-5 years) for Group 1; 36 months (age range 1-4 years) for Group 2, and 24 months (age range 1-3 years) for Group 3.
The two primary dependent measures for this analysis were:
1. The number of entries made in the health records (Veterinary Treatments).
2. The number of Days of Therapy received by each subject.
In addition, Veterinary Treatments were divided into three mutually exclusive categories relating to the cause of the problem: diarrhoea and dehydration, trauma (e.g. a bite wound) and miscellaneous problems (e.g. dermatitis, ocular inflammations and abscesses). The number of Days of Therapy was defined as the number of days that the monkey received a veterinary intervention. In most cases, this represented the administration of some medication or the changing of bandages.
Chi-square analyses were performed to determine whether Veterinary Treatments and Days of Therapy were randomly distributed across enrichment and housing conditions.
For descriptive purposes, the raw numbers of Veterinary Treatments and Days of Therapy were divided by the number of days that subjects were participating in the study. The number of days of a housing condition was multiplied by the number of monkeys in that condition each day to obtain a quantity referred to as the number of Monkey Days for that condition. For example, 32 monkeys living in any condition for one year (365 days) account for 11,680 Monkey Days (32 x 365). There were approximately the same number of Monkey Days for each setting. All three groups were assessed while alone and paired, whereas only Groups I and 2 were studied while living in groups, but Group 1 was studied for two years while group-housed. Rates of veterinary treatment were determined by dividing the number of treatments by the number of Monkey Days.
Results
There were few interventions and days of treatment for this group of 98 monkeys across the four years studied. However, it is possible that the data drawn from the animal health records underestimated the number of health problems, particularly for analyses of diarrhoea and dehydration. The first step in the remedy of diarrhoea was simply to alter the affected animal's diet (no enrichment foods for enriched subjects, no fruit for control subjects), without recording this as a Veterinary Treatment in the animal's records. Therefore, such dietary manipulations were not included in the analysis.
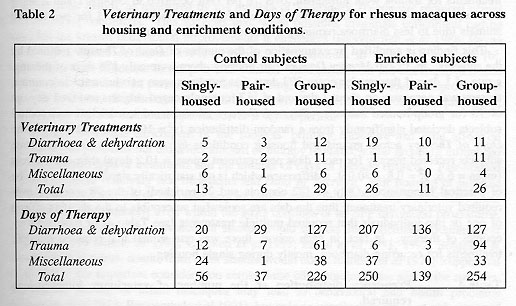
There were 111 Veterinary Treatments recorded for the 98 monkeys (see Table 2). Forty-eight occurred for control subjects and 63 occurred for enriched subjects. Neither this difference (x sq= 2.02, P>0.05) nor the difference across combinations of enrichment and housing conditions (2 x 3) was significant (x sq= 3.99, P>0.05). Treatments were infrequent, but they were not randomly distributed when examined across housing conditions only (x sq= 19.7, P<0.001). Subjects required treatment once every 909 Monkey Days while singly-caged; once every 2,104 Monkey Days while pair-housed, and once every 637 Monkey Days while group-housed (see Table 3). The combined figures indicate that when at full capacity (98 monkeys in the three housing conditions), there was on average one treatment every ten days.
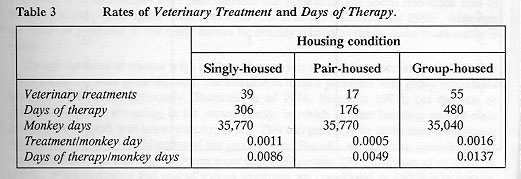
Over half (60/111) of all Veterinary Treatments were related to diarrhoea and dehydration and of these 60 occurrences, 24 took place while subjects were housed alone. Although treatments for trauma were infrequent, over 78 per cent occurred in Groups 1 and 2 while they were group-housed. There was relatively little intervention needed for pair-housed animals (due to less diarrhoea requiring treatment and little trauma).
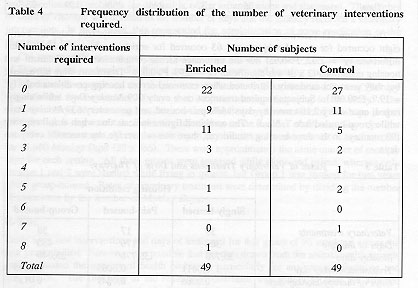
This finding is amplified by examination of the number of Days of Therapy required by the subjects. In 35,770 Monkey Days of pair housing, there were only 176 days of therapy, a rate of 1 day of therapy for every 203 days that monkeys were pair-housed. In contrast, therapy was necessary on about 1 day out of 117 for singly-caged subjects and on 1 day out of 73 for group-housed subjects. Days of Therapy administered to enriched and control subjects deviated significantly from a random distribution (y sq= 109.2, P<0.001) as did the Days of Therapy across groups and housing conditions (x sq= 84.1, P<0.001). Enriched subjects received therapy for more days per treatment (mean = 10.2 days) than did controls (mean = 6.6, x sq= 0.8, P>0.05), a difference which is not statistically significant, but may be of practical importance. Only 49 (22 controls and 27 enriched) of the 98 monkeys ever required veterinary treatment; thus the data are somewhat susceptible to the skewing effects of one or two individuals that required multiple treatments (see Table 4) or received long courses of therapy. In fact, in each cohort, there was one animal that required multiple treatments for recurring diarrhoea, mostly during single housing.
Discussion
Inanimate enrichment did not adversely affect the health of rhesus monkeys, nor did it improve their health, as measured by the number of Veterinary Treatments required across three housing conditions. This suggests that, when possible, inanimate enrichment can be used for the known behavioural. improvements it engenders (Bloomsmith et al 1988; Bayne et al 1991; Byrne & Suomi 1991; Schapiro & Bloomsmith in press [a], in press [b], submitted), with little cost to the primates' health. That enrichment did not improve health along with behaviour is perhaps a little surprising, given the behavioural improvements referred to above. But the overall health of the derived subjects appeared to be quite good; there were only 111 treatment episodes over a 4-year period.
Whereas inanimate enrichment of the subjects' primary enclosure had little effect on animal health problems but did affect amount of therapy required, social grouping influenced the number and type of treatments and the number of days of therapy required. Pair-housed animals required relatively few treatments and Days of Therapy. Pair housing of juvenile rhesus monkeys may be an effective way to satisfy many husbandry and behavioural goals in a cost-effective manner, even within the strict confines of an SPF derivation programme. Although the results from a number of studies would support this conclusion (Reinhardt et al 1987, 1988; Line et al 1990; Crockett et al 1991; Eaton et al 1991, 1992; Schapiro & Bloomsmith 1994), results from other studies would not (Chamove et al 1973; Ruppenthal et al 1991). An important consideration across these other studies is the age of the subjects. Continuous pairing of infant macaques may lead to behavioural and health problems (Chamove et al 1973; Ruppenthal et al 1991), but pairing yearlings or other combinations of non-infants and infants may be more successful (Brandt & Mitchell 1973; Reinhardt et al 1988; Eaton et al 1992; Schapiro & Bloomsmith 1994). In situations where breeding colonies are being established, socialization via pairing at appropriate times may satisfy objectives related to both management and psychological well- being (Mason 1991).
Single housing, especially immediately following separation from the natal group, may necessitate frequent treatments and therapy. In this study, treatment and therapy for diarrhoea and dehydration were most common during the year of single housing. One potential interpretation of the incidence of diarrhoea-related disorders in subjects housed alone is that these animals were under stress due to the separation and social restriction of this housing condition. Previous studies have demonstrated that separation and social restriction lead to behavioural and physiological stress responses (Coe et al 1983; Levine et al 1985). Although the observed problems in our study cannot be directly related to stress, some relationship is likely.
Though episodes of trauma were neither frequent nor severe, most occurred while Group 1 and 2 subjects were group-housed. An increase in trauma problems with group formations has been documented previously (Bernstein et al 1974; Bernstein 1991), but the rate of groupmate-induced wounding in the present study, in which 16 new breeding and all-male groups were formed, was lower than expected. This can probably be attributed to the young age and the similar social histories of the groupmates. Socially experienced, juvenile rhesus macaques form into new social groups most easily (Bernstein 1991; Schapiro et al 1992, in press). When diarrhoea and miscellaneous treatment are considered in addition to trauma, it becomes apparent that rates of treatment and the number of Days of Therapy were highest for subjects while group-housed. Whereas desirable increases in behavioural complexity and cost-effectiveness of husbandry efforts may be more readily achieved for rhesus monkeys housed in groups, the potential for increased veterinary care must also be considered.
The analyses across housing conditions were potentially confounded by the age of the monkeys. Due to the nature of the SPF derivation programme being followed, all singly-caged subjects were between one and two years of age; all pair-housed subjects were between two and three years of age, and all group-housed subjects were between three and four years of age. Subjects' ages affect both their response to separation (Mineka & Suomi 1978; Levine et al 1985; Champoux et al 1989) and the ease with which they can be integrated into new social groups (Bernstein 1991). Future investigations will be aimed at eliminating this confounding factor, by studying a modified derivation programme that includes group housing of 1 to 3 year-olds.
Although control and enriched subjects required similar numbers of Veterinary Treatments that were distributed comparably across the causes of the health problems, the two groups differed significantly in the number of Days of Therapy they required. It is unclear why enriched subjects received therapy for an average of over 10 days per treatment while control subjects received less than 7 days of therapy per treatment. It would appear that enriched subjects were more ill than controls, requiring longer courses of therapy to recover. This implies that where environmental enrichment is to be used, provisions should be made for potentially higher therapy requirements.
Only 50 per cent of the monkeys studied ever required a veterinary treatment, attesting to the good health of the animals as they passed through the SPF derivation programme. Digestive problems, as evidenced by loose stool, were more frequent than the data indicated, however. The first treatment for these problems was to remove food items from the diet that might be causing digestive distress. These manipulations were not recorded
This study demonstrates that there may be advantages to pair housing juvenile rhesus macaques. Veterinary care requirements were lower for pairs than either single or group housing conditions, yet subjects still had the social enrichment opportunity of a peer. Diarrhoea-related problems typical of singly-housed animals and trauma-related problem,, typical of group-housed animals were not as prominent in the pairs. While nonsocia enrichment neither enhanced nor diminished animal health as measured by the frequency o treatment, it did increase the amount of therapy required, suggesting that enriched animal; took longer to recover from a health problem than did controls.
Animal welfare implications
When designing enrichment and housing programmes for captive primates, one must consider how such programmes may affect the primates' physical and psychological health. Both inanimate and social forms of enrichment can support behavioural improvements and ma alter the risk of injury or disease. Assessing the effects of inanimate and social enhancement of the environment on the veterinary requirements of captive primates is necessary to develop cost-effective enrichment and housing procedures that promote both the psychological an physical welfare of animals.
Acknowledgments
We thank the primate section staff for their assistance and record-keeping vigilance. Thanks also to K Flynn at Scientific Publications, M D Anderson Cancer Center, for editing advice. Animals are maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current United States Department of Agriculture; Department of Health and Human Services, and National Institutes of Health regulations and standards. Financial support for this project came from the National Institutes of Health/NCRR grants U42-RRO5080 and R01- RR05092.
References
Bayne K A L, Dexter S L, Hurst J K, Strange G M and Hill E E 1993 Kong toys for laboratory primates: are they really an enrichment or just fomites? Laboratory Animal Science 43: 78-85
Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F and Suomi S 1991 The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology 23: 23-25
Bernstein I S 1991 Social housing of monkeys and apes: group formations. Laboratory Animal Science 41: 329-333
Bernstein I S, Gordon T P and Rose R M 1974 Factors influencing the expression of aggression during introductions to rhesus monkey groups. In Holloway R L (ed) Primate Aggression, Territoriality and Xenophobia - A Comparative Perspective pp 211-240. Academic Press: New York, NY
Bloomsmith M A, Alford P L and Maple T L 1988 Successful feeding enrichment for captive chimpanzees. American Journal of Primatology 16: 155-164
Bloomsmith M A, Keeling M E and Lambeth S P 1990 Videotapes: environmental enrichment for singly-housed chimpanzees. Lab Animal 19(1): 42-46
Brandt E M and Mitchell G 1973 Pairing preadolescents with infants (Macaca mulatta) Developmental Psychology 8: 222-22
Brent L, Lee D R and Eichberg JW 1989 Evaluation of two environmental enrichment devices for singly caged chimpanzees (Pan troglodytes). American Journal of Primatology (Supplement) 1: 65-70
Bryant C E, Rupniak N M J and Iverson S D 1988 Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys. Journal of Medical Primatology 17: 257-269
Byrne G D and Suomi S J 1991 Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. American Journal of Primatology 23: 141-151
Chamove A S, Anderson J R, Morgan-Jones S C and Jones S P 1982 Deep woodchip litter: hygiene, feeding, and behavioral enhancement in eight primate species. International Journal of Studies of Animal Problems 3: 308-318
Chamove A S, Rosenblum L A and Harlow H F 1973 Monkeys (Macaca mulatta) raised only with peers. A pilot study. Animal Behaviour 21: 316-325
Champoux M, Coe C L, Schanberg S M, Kuhn C M and Suomi S J 1989 Hormonal effects of early rearing conditions in the infant rhesus monkey. American Journal of Primatology 19: 111-117
Clarke M R, Koritnik D R, Martin L N and Baskin G B 1989 Cage enrichment, physiology, and behavior in nursery-reared rhesus monkeys. American Journal of Primatology (Supplement) 1: 53-57
Coe C L, Glass J C, Wiener S G and Levine S 1983 Behavioral, but not physiological, adaption to repeated separation in mother and infant primates. Psychoneuroendocrinology 18: 401-409
Crockett C M, Bowden D M, Bowers C L and Sackett G P 1991 Social pairing of longtailed macaques with preferred, nonpreferred, and randomly assigned cagemates. American Journal of Primatology 24: 94-95
Eaton G G, Kelley S T and Axthelm M 1991 Assessment of psychological well-being in paired rhesus females. American Journal of Primatology 24: 97-98
Eaton G G, Kelley S T, Axthelm M and Iliff-Sizemore S A 1992 Reproductive parameter of psychological well-being in paired female rhesus. American Journal of Primatology 27: 26-27
Erwin J 1977 Factors influencing aggressive behavior and risk of trauma in the pigtai macaque (Macaca nemestrina). Laboratory Animal Science 27: 541-547
Erwin J and Sackett G P 1990 Effects of management methods, social organization, and physical space on primate behavior and health. American Journal of Primatology 20: 23 30
Levine S, Johnson D F and Gonzales C A 1985 Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behavioral Neuroscience 99: 399-410
Line S W and Morgan K N 1991 The effects of two novel objects on the behavior of single- caged adult rhesus macaques. Laboratory Animal Science 41: 365-369
Line S W, Morgan K N, Markowitz H, Roberts J A and Riddel M 1990 Behavior responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Laboratory Primate Newsletter 29(4): 1-5
Line S, Morgan K, Markowitz H and Strong S 1989 Influence of cage size on heart rate and behavior in rhesus monkeys. American Journal of Veterinary Research 50: 1523-1526
Mason W A 1991 Effects of social interaction on well-being: development aspects. Laboratory Animal Science 41: 323-328
Mincka S and Suomi S J 1978 Social separation in monkeys. Psychological Bulletin 8: 1376-1400
Molzen E M and French J A 1989 The problem of foraging in captive Callitrichid primates: behavioral time budgets and foraging skills. In Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 89-101. Noyes Publications: Park Ridge, New Jersey
Novak M A and Drewsen K H 1989 Enriching the lives of captive primates: issues and problems. In Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 161-182. Noyes Publications: Park Ridge, New Jersey
Novak M A and Suomi S J 1988 Psychological well-being of primates in captivity. American Psychologist 43: 765-773
O'Neill P L, Novak M A and Suomi S J 1991 Normalizing laboratory-reared rhesus macaque (Macaca mulatta) behavior with exposure to complex outdoor enclosures. Zoo Biology 10: 237-245
Reinhardt V, Houser W D, Eisele S G and Champoux M 1987 Social enrichment of the environment with infants for singly caged adult rhesus monkeys. Zoo Biology 6: 365-371
Reinhardt V, Houser D, Eisele S, Cowley D and Vertein R 1988 Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. American Journal of Primatology 14: 135-140
Ruppenthal G C, Walker C G and Sackett G P 1991 Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages.American Journal of Primatology 25: 103-113
Schapiro S J and Bloomsmith M A 1994 Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. American Journal of Primatology 32: 159-170
Schapiro S J and Bloomsmith M A 1995 Behavioral effects of enrichment on singly-housed, yearling rhesus monkeys: an analysis including three enrichment conditions and a control group. American Journal of Primatology 35: 89-101
Schapiro S J, Bloomsmith M A, Kessel A L and Shively C A 1993a. Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science 37: 251-263
Schapiro S J, Bloomsmith M A, Porter L M and Suarez S A 1993b Housing conditions and/or age more strongly affect the behavior of young rhesus monkeys than does inanimate enrichment. American Journal of Primatology 30: 346 (Abstract)
Schapiro S J, Brent L Y, Bloomsmith M A and Satterfield W C 1991 Enrichment devices for nonhuman primates. Lab Animal 20(6): 22-28
Schapiro S J and Kessel A L 1993 Weight gain among juvenile rhesus macaques: a comparison of enriched and control groups. Laboratory Animal Science 43: 315-318
Schapiro S J, Lee-Parritz D E, Taylor L L, Watson L, Bloomsmith M A and Petto A 1992 Behavioral management of specific pathogen-free (SPF) rhesus macaques. Paper presented at the XIVth Congress of the International Primatological Sociey, Strasbourg, France
Schapiro S J, Lee-Parritz D E, Taylor L L, Watson L, Bloomsmith M A and Petto A (1994 Behavioral management of specific pathogen-free (SPF) rhesus macaques: group formation, reproduction, and parental competence. Laboratory Animal Science 44: 229-234
Voss W R, Buchl S J, Keeling M E, Hilliard J, Lerche N, Schapiro S J and Bloomsmith M A 1991 Derivation strategy for establishing a Macaca mulatta (rhesus) colony specifically pathogen-free of Herpes B virus and simian retrovirus. AALAS Bulletin 30: 18-19
This article originally appeared in Animal Welfare 3: 25-36 (1994)
Reprinted with permission of the publisher.