S P Lambeth and M A Bloomsmith
The University of Texas M D Anderson Cancer Center Science Park,
Department of Veterinary Sciences, Route 2, Box 151-B1, Bastrop, Texas 78602, USA
Abstract
In the wild, chimpanzees spend most of their time foraging, so any device that stimulates this behaviour in captivity could potentially be effective enrichment. A simple grass foraging device constructed of a polyvinyl chloride (PVC) pipe cut in half lengthwise and planted with rye grass seed was designed to allow captive chimpanzees living in non- grassy enclosures to exhibit foraging similar to that of their wild counterparts. The grass containers were attached to the outside of six different enclosures. Observational data were collected on 14 adult chimpanzees (eight females, six males) within groups of either two or four members. A total of 54 hours of behavioural observations were conducted and comparisons were made across three conditions: baseline; grass container; grass container with extra foraging material (one half cup of sunflower seeds). Subjects used the grass container 4.0 percent of their time, but for 19.8 per cent of their time when the grass container [was loaded] with extra foraging material. There was no statistical evidence of habituation to the device. Overall, the grass container only increased time spent foraging when it contained additional food items. Since behavioral benefits associated with this device are few, its potential application is limited.
Keywords: animal welfare, chimpanzees, environmental enrichment, foraging, psychological well-being
Introduction
Feeding and foraging make up the most time-consuming activity of wild chimpanzees (Wrangharn 1977; Goodall 1986), while foraging in captivity is generally much less time consuming. In the design of feeding enrichment procedures for captive chimpanzees, it may be useful to use the feeding behaviour of their wild counterparts as a model to simulate (Line 1987; Novak & Suomi 1988; Bloomsmith 1989). Primatologists have suggested that the great reduction in feeding time in captivity is responsible for some behavioural management problems such as certain stereotypical behaviour (Fritz & Fritz 1979; Maple 1980).
Several devices and procedures have been studied that provide primates with opportunities to exhibit natural foraging behaviours in captivity (Nash 1982; Tripp 1985; McGrew et al 1986; Makiet al 1989; Hayes 1990). Some feeding enrichment strategies have increased activity, reduced agonism (Chamove & Anderson 1979; Bloomsmith et al 1988; Boccia 1989; Brent & Eichberg 1991) and reduced pathologic behaviours (Chamove et al 1982; Gould & Bres 1986; Bloomsmith et al 1988; Bayne et al 1991). It is important to quantitatively evaluate the effects of these enrichment strategies on a variety of behavioural pattern to report the results, either positive or negative, so that those designing enrichment programmes can make informed decisions about the value of various feeding enrichment procedures.
The current study quantifies the use of a grass container as a foraging device for captive chimpanzees living in enclosures without a foraging substrate. At The University of Texas M D Anderson Cancer Center, chimpanzees living in large, outdoor corrals spend two per cent of their time feeding on grass or foraging for items in the grass (unpublished dada). Seeding a ground cover with small foods has successfully increased foraging in several primate species (Chamove & Anderson 1979, 1982; Bloomsmith et al 1988; Boccia 1989). The use of a grass substrate helps to simulate conditions in the wild and also allows hiding small foods to promote foraging. However, since some captive primates do not have access to a grassy substrate, a device was designed to offer an opportunity to forage in grass. This study was completed to determine whether this device increased species-typical foraging behaviour, and to assess any other behavioural effects.
Methods
Subjects and housing
The subjects were eight female and six male adult chimpanzees (Pan troglodytes) housed at the Science Park chimpanzee breeding facility of The University of Texas M D And Cancer Center. Subjects were housed in six different enclosures with one group of four animals and the remaining subjects in pairs. Subjects lived in indoor/outdoor runs, measuring 2.4 x 6.1 x 2.4m with a concrete floor, resting boards, barred ceilings, and cinder block or chain link fencing separating the runs. The group of four subjects lived in two of these indoor/outdoor runs. During the course of the study, animals were given browse, manipulable objects such as balls, cardboard boxes and plastic barrels, and a variety of feeding enrichment opportunities according to a daily enrichment programme.
Apparatus
A 7.69cm diameter, schedule 40 (thickness) polyvinyl chloride (PVC) pipe 61.5cm in length was capped at both ends and cut in half lengthwise. Two 1.28cm drain holes were cut in the bottom of each pipe (see Figure 1). Supplies for each grass container cost about US$5.67. The containers were filled with potting soil and planted with rye grass seed. When the reached about 8cm in height (5 to 8 days after planting), two J-bolts were used to anchor the grass container to the chain link fencing on the front panel of each subject's enclosure. The container was positioned 62cm from the floor, allowing the subjects to use the planter a level. The expected behaviour was for a chimpanzee to insert two or more fingers through the chain link fencing to forage or pick grass, without being able to destroy the device.
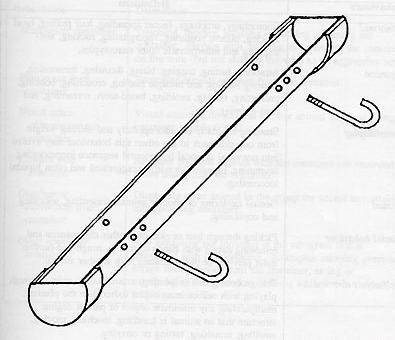
Procedures
The first condition of the study was conducted as a baseline with no grass container present. Since the enclosures had concrete floors the subjects had no opportunity to forage in a substrate. The second condition consisted of attaching the container with only grass to the front panel of the chain link fencing of the subjects' enclosures. In the third condition, approximately one half cup of sunflower seeds was distributed in the grass, providing extra foraging material. These three study conditions were concurrently completed over a four month period. The conditions were alternated on an unpredictable schedule with the condition determined by the experimenter prior to each session. This methodology eliminates the potential confound of time which would exist if the study conditions were completed sequentially.
Data collection
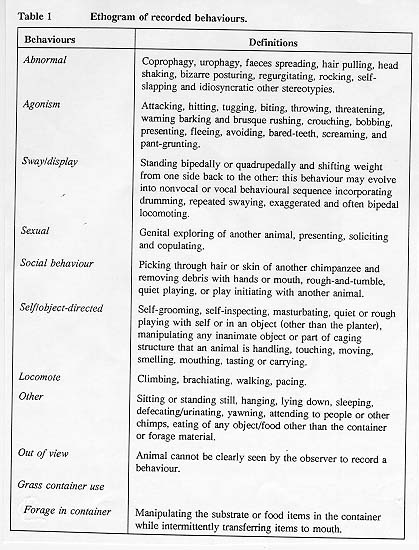
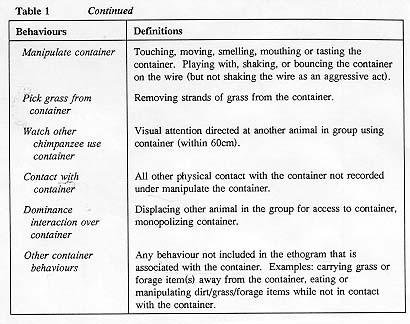
A total of 108 thirty-minute observation sessions were completed by two observers between June and October of 1991. An all-subjects scan sampling data collection technique was used, with recordings made every 10 seconds. This inter-sample interval generated 180 data points per subject for each observation session, and was judged to be the shortest interval possible which still maintained acceptable levels of inter-observer reliability. Six observations were collected for each of the six subject groups in each of the three study conditions for a total of 54 hours of data. Subjects were not exposed to the grass containers outside of these observation periods so they had a total of 12 exposures to the containers - six times with grass alone and six times with extra foraging material. The order in which the groups were observed was randomized. Observations were conducted between 0730h and 1130h in the morning. Generally, 10 tests a week were collected. Subjects had access to both the indoor and outdoor portions of the enclosures during 71 per cent of the tests; during 29 per cent of the tests they were restricted to the outside area of the enclosure while the indoor enclosures were being cleaned. Restriction to the outside portion of the enclosure had no effect on grass container use as measured by analysis of variance (P>0.05), so no further analysis of that factor was undertaken and data were collapsed for that factor in the other analyses completed Inter-observer reliability was 92 per cent when measured by a Kappa coefficient (Martin & Bateson 1986). See Table 1 for definitions of the behaviours recorded.
Analysis
The nine categories of behaviour indicated in Table 1 were used for analysis: abnormal; agonism; sway/display; sexual; social behaviour; self/object-directed (other than grass container); locomote; other and grass container use. The mean percentage of the data points were generated for each of these nine behavioural categories, for each of the 14 subjects within each of the three experimental conditions. These percentage scores served as the unit of measure for statistical analysis.
A Pearson's correlation analysis of container use against time was conducted to test for habituation of container use. A multivariate analysis of variance (MANOVA) for repeated measures was used to analyse the grass container's effect on the mean percentage scores for the nine behavioural. categories. ANOVA was used to measure whether there was a sex difference in the amount of container use. To control for Type I errors with the large number of statistical tests performed, significance was defined by P<0.001.
Results
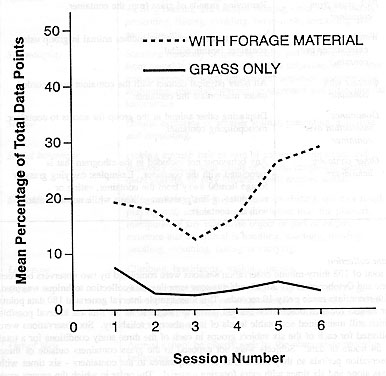
A Pearson's correlation analysis of grass container use against time for each of the two experimental conditions was used to test for habituation. No significant correlations were found (P>0.05), indicating that the subjects did not habituate either to the container with grass only or to the container with extra foraging material.
A MANOVA for repeated measures revealed an overall significant effect of the grass container among the three conditions (Wilks' Lambda = 0.30; F = 3.41; df = 18,74). Posthoc pairwise comparisons revealed that the container with grass did not significantly affect the subjects' behaviour as compared to baseline. However, the container with extra foraging material did significantly affect the subjects' behaviour when compared with the baseline condition as measured by a MANOVA (Wilks' Lambda = 0.42; F = 5.73; df = 9,37). The univariate tests showed a significant increase in container use (F = 45.40; df = 1,45) when the container with extra foraging material was available. (See Table 2 for mean percentages of behaviours.)
The container with extra foraging material also significantly affected the subjects' behaviour when compared to the container with grass only, as measured by a MANOVA (Wilks' Lambda = 0.46; F = 4.93; df = 9,37). The univariate tests showed higher amounts of container use (F = 29.18; df = 1,45) when the grass container had extra foraging material (see Table 2 for mean percentages of behaviours; Figure 2).
An ANOVA conducted on container use revealed no influence of sex when subjects had the container with grass only or when they had the container with extra forage material.
Table 2. Mean percentages of behaviour in the three conditions of the study.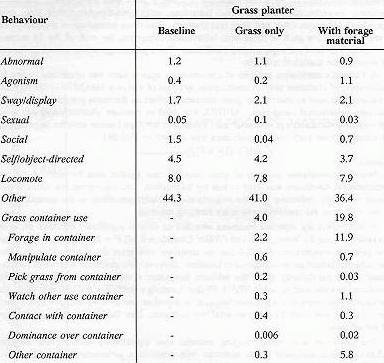
Discussion
The results of this study indicate that only when the grass container had extra foraging material did it effectively change chimpanzee behaviour in a species-appropriate direction by increasing foraging time. The container with grass alone was ineffective in changing the chimpanzees' behaviour. In many enrichment studies subjects habituate to the device or material and use declines over the course of the study (Bryant et al 1988; Paquette & Prescott 1988; Bloomsmith et al 1990; Pruetz & Bloomsmith 1992). In this study subjects did not habituate to the grass container (with or without extra foraging material) over six different 30-minute exposures. This finding concurs with some other foraging studies that have also shown consistent use over time (Bloomstrand et al 1986; Bryant et al 1988; Bloom & Cook 1989; Boccia 1989; Bayne et al 1991), so the container may be practical for long-term implementation. Both males and females consistently used the device.
The amount of use of the foraging device when it contained extra foraging material (19.8% of the time) in this study generally corroborates the level of foraging found in other studies of enrichment devices to stimulate foraging (Maki et al 1989; Hayes 1990; Brent & Eichberg 1991; Bayne et al 1991), but no other behavioral consequences were found. Some other studies have reported more widespread effects of feeding devices or techniques beyond influences on foraging. Chamove and colleagues (Chamove & Anderson 1979; Chamove et al 1982) documented influences on aggression, inactivity, play and other affiliative behaviour by using woodchip litter sometimes seeded with food. Bloomsmith et al (1988) measured lower levels of agonism and abnormal behaviour when a feeding enrichment programme was implemented. Maki et al (1989) developed a food puzzle device to simulate termite fishing and found an increase in aggression associated with its use in a large group of chimpanzees. Hayes (1990) constructed a hanging feeder filled with hay and food items for capuchin monkeys which decreased inactivity and locomotion while increasing foraging. A foraging/grooming board reduced abnormal behaviour among rhesus monkeys (Bayne et al 1991). Brent & Eichberg (1991) provided a puzzleboard for captive chimpanzees and found reductions in inactivity, aggression, affiliation, and self-directed behaviour.
Even though there were high levels of container use in one condition of the current study, abnormal behaviour was not moderated. An explanation may be that the baseline value for abnormal behaviour, consisting mainly of coprophagy, was low (1.2% of their time). The subjects have been participating in a daily enrichment programme since long before the study began, and perhaps the limit of influence that enrichment could have had on abnormal behaviour patterns in these animals in their current housing situation had already been reached. Some other enrichment studies have also found abnormal behaviour to be resistant to change (Bloomstrand et al 1986; Brent & Eichberg 1991; Lambeth & Bloomsmith 1992).
The success of the enrichment device described in this paper was limited to increasing species-typical foraging behaviour while maintaining a higher level of consistent use by chimpanzees of both genders when it contained extra foraging material. This device cannot be recommended to address other behavioural. problems such as moderating aberrant behaviour or stimulating prosocial. interactions. It is important to report the evaluation of this device even though it did not extensively alter behaviour, because the limitations of enrichment devices should be understood if the devices are to be used appropriately in an enrichment programme. Because of the limitations of this device, other feeding devices might be easier or more effective to use in some circumstances. However, the device was inexpensive and well-suited for captive chimpanzees in this housing situation in terms of safety, ability to be sanitized and ease of installation, although it requires several days for growing the grass. It may be more practical for example, to use this same device as a container for woodchips that could be seeded with food since the subjects did not benefit from the grass alone. This device may be appropriate for other primate species as it could be attached to the outside of many types of enclosures. However, the limited behavioural. benefits associated with this device may mean that other feeding enrichment devices should first be considered when trying to design an enrichment programme that would alter many behaviours in species- typical directions, thereby improving psychological well-being.
Animal welfare implications
One method of assessing animal welfare is to objectively measure behavioural. indices of psychological health. Some behavioural scientists have argued that the behaviour of wild primates should serve as a model such that the behaviour of captive primates would ideally approximate that of wild primates (Line 1987; Novak & Suomi 1988; Bloornsmith 1989). Many techniques exist to enrich captive primates by altering their environments, some of which can be shown to be beneficial by increasing species-typical behaviour. This study quantifies the use of a foraging enrichment device to measure whether it can change the behaviour of captive chimpanzees to more closely resemble that of chimpanzees in the wild.
Acknowledgments
The authors would like to thank Kyle Burk for assisting in data collection and Sharon Powell for computer programming. We also thank Carolyn Randall for manuscript preparation and Kevin Flynn for manuscript editing. This project was supported by the National Institute,, of Health/National Center for Research Resources grants R01- RR03578 and U42-RRO358S and contract 263-88-C-0248. Animals are maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current United States Department of Agriculture, Department of Health and Human Services, an( National Institutes of Health regulations and standards.
References
Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F and Suomi S 1991 The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with foraging/grooming board. American Journal of Primatology 23: 23-25
Bloom K R and Cook M 1989 Environmental enrichment: behavioral responses of rhesus to puzzle feeders. Lab Animal 18(5): 25-31
Bloomsmith M A 1989 Feeding enrichment for captive great apes. In Segal E F (ed Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 336 356. Noyes Publications: Park Ridge, NJ
Bloomsmith M A, Alford P L and Maple T L 1988 Successful feeding enrichment of captive chimpanzees. American Journal of Primatology 16:- 155- 164
Bloomsmith M A, Finlay T W, Merhalski 3 and Maple T L 1990 Rigid plastic balls a enrichment for captive chimpanzees. Laboratory Animal Science 40: 319-322
Bloomstrand M, Riddle K, Alford P and Maple T L 1986 Objective evaluation of behavioral enrichment device for captive chimpanzees (Pan troglodytes). Zoo Biology 293-300
Boccia M L 1989 Preliminary report on the use of a natural foraging task to reduce aggression and stereotypies in socially housed pigtail macaques. Laboratory Primal Newsletter 28(1): 3-4
Brent L and Eichberg J W 1991 Primate puzzleboard: a simple environmental enrichment device for captive chimpanzees. Zoo Biology 10: 353-360
Bryant C E, Rupniak N M J and Iversen S D 1988 Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys. Journal of Medical Primatology 17: 257-269
Chamove A S and Anderson J R 1979 Woodchip litter in macaque groups. Journal of the Institute of Animal Technicians 300: 69-74
Chamove A S, Anderson J R, Morgan-Jones S C and Jones S P 1982 Deep woodchip litter: hygiene, feeding and behavioral enhancement in eight primate species. International Journal for the Study of Animal Problems 3: 308-318
Fritz P and Fritz J 1979 Resocialization of chimpanzees. Journal of Medical Primatology 8: 202-221
Goodall J 1986 The Chimpanzees of Gombe: Patterns of Behavior. Belknap: Cambridge
Gould E and Bres M 1986 Regurgitation and reingestion in captive gorillas: description and intervention. Zoo Biology 5: 241-250
Hayes SL 1990 Increasing foraging opportunities for a group of captive capuchin monkeys (Cebus capucinus). Laboratory Animal Science 40: 515- 519
Lambeth S P and Bloornsmith M A 1992 Mirrors as enrichment for captive chimpanzees (Pan troglodytes). Laboratory Animal Science 42: 261-266
Line S W 1987 Environmental enrichment for laboratory primates. Journal of the American Veterinary Medical Association 190: 854-859
McGrew W C, Brennan J A and Russell J 1986 An artificial gum-tree' for marmosets (Callithrix jacchus). Zoo Biology 5: 45-50
Maki S, Alford P L, Bloomsmith M A and Franklin J 1989 Food puzzle device simulating termite fishing for captive chimpanzees (Pan troglodytes). American Journal of Primatology Supplement 1: 71-78
Maple T L 1980 Chimpanzee reproduction, rearing, and rehabilitation in captivity. Report submitted to the Ad Hoc Task Force, National Chimpanzee Breeding Program, Tanglewood, NC
Martin P and Bateson P 1986 Measuring Behaviour: An Introductory Guide pp 86-97. Cambridge University Press: Cambridge
Nash V J 1982 Tool use by captive chimpanzees at an artificial termite mound. Zoo Biology 1: 211-221
Novak M A and Suomi S J 1988 Psychological well-being of primates in captivity. American Psychologist 48: 765-773
Paquette D and Prescott J 1988 Use of novel objects to enhance environments of captive chimpanzees. Zoo Biology 7: 15-23
Pruetz J D and Bloomsmith M A 1992 Comparing two manipulable objects as enrichment for captive chimpanzees (Pan troglodytes). Animal Welfare 1: 127-137
Tripp J K 1985 Increasing activity in captive orangutans: provision of manipulable and edible materials. Zoo Biology 4: 225-234
Wrangham R W 1977 Feeding behavior of chimpanzees in Gombe National Park, Tanzania. In Clutton-Brock T H (ed) primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys and Apes pp 503-538. Academic Press: New York, NY
Printed with permission of the publisher.
This article originally appeared in Animal Welfare 1994, 3: 13-24