Jaylan S. Turkkan
Department of Psychiatry and Behavioral Sciences, Division of Behavioral Biology,
The Johns Hopkins University School of Medicine, Baltimore
Accepted for publication May 5, 1989.
Adult male baboons were behaviorally conditioned to extend an arm outside of the living cage and to accept repeated cuff inflations for manual auscultatorv blood pressure measurements. Frequency distributions of systolic and diastolic blood pressure for both normotensive and renovascular hypertensive baboons generally were normally distributed. The procedure accurately tracked rapid changes in blood pressure after oral administration of antihypertensive drugs. Advantages over direct arterial cannulation for blood pressure measurement during extended, chronic experiments are discussed.
Key words: Indirect blood pressure - Auscultatory - Systolic and diastolic blood pressure - Nonhuman primates - Papio sp. - Hypertension - Antihypertensive medication
INTRODUCTION
A number of procedures have been developed and implemented in recent years that widen the range of methodological options for conducting experiments with nonhuman primates by obviating the need for chronic surgical implantation of catheters. Blood pressure (BP) measurement In animal studies, and in nonhuman primates in particular. has been conducted almost exclusively through the use of chronic indwelling catheters connected to external transducers [11]. In such a system. a heparinized fluid-filled catheter is surgically inserted during anesthesia into a large artery or directly into the ventricle and tunneled subcutaneously to an exit site. The catheter is then protected from the awake animal either through the use of restraint chairs or, more recently, by use of harness-tether or harness-telemetry systems [2,5,10,11.13]. For individual BP measurement probes, catheters have been temporarily introduced into an artery and connected to a pressure transducer during ketamine anesthesia, after which the catheter is withdrawn (8]. Catheter microtip manometers have also been used. but calibration has been reported to shift, and they have not been recommended for use in chronic experiments [11].
We have previously reported the development of a swivel-tether-harness system [7] for the measurement of direct arterial BP in awake adult male baboons with use of a miniature transducer inserted into a backpack [ 131. Some disadvantages of direct arterial measurement. also frequently addressed in reports of clinical BP measurement, led to the development of alternative methodologies for measuring BP in awake baboons. For example, because BP is measured through a fluid-filled system, changes in the frequency response of the transducer and phenomena of fluid dynamics such as ringing [6] have often led to misinterpretation of systolic BP and incorrect categorization of patients as hypertensive. In extended studies with animals, repeated and unpredictable loss of catheter patency resulting from blood clotting at the tip of the catheter or from infections at surgical exit sites have led to interruptions of chronic experiments in our own laboratories. Catheter removal. recovery, surgical recatheterization, and further postsurgical recovery have in the past involved months of experimental delay, resulting in fewer subjects studied than is advisable for statistical power or for demonstration of experimental control. Moreover. surgery for arterial catheterization can disrupt hormonal and cardiovascular levels sometimes for weeks after surgery until BP baselines are recovered. Finally. restricted body movement and access to body parts for self-grooming may lead to behavioral and physiological states that may not be representative of the resting primate. For example. in a recent study of cynomolgus monkeys, elevated heart rates were observed after each animal was fitted with a nylon-mesh jacket and tether that protected an arterial catheter. treatment with the beta-blocking agent propranolol reduced heart rates to pretethering levels [1].
We report here a method of obtaining auscultatory, indirect BP measurements from the awake nonhuman primate with use of behavioral conditioning techniques. The use of this method has eliminated some of the disadvantages of using arterial catheterization to obtain blood pressure measurements in chronic studies of antihypertensive medications.
MATERIALS AND METHODS
Subjects
Six adult male baboons (Papio cynocephalus, hamadryas, and anubis) approximately 4-5 years of age served as subjects. Animals ranged in weight between 14 and 28 kg and were housed in individual living cages (LWH 48 X 35 X 56" for >25 kg animals and 38 X 32 X 46" for £ 25kg animals), with an opening for arm extension, and a seating bench. Water was continuously available.
Baboons DE, HA, and ME were shipped to this laboratory approximately 3 months after unilateral left renal artery stenosis (two-kidney, one-clamp) was performed at the Southwest Foundation for Biomedical Research [8] to induce a hypertensive state. After arrival in the laboratory, all baboons were trained to present their arms for auscultatory systolic (SBP) and diastolic (DBP) blood pressure measurement (see below).
These baboons are currently engaged in a project investigating the effects of standard orally administered antihypertensive drugs on visual (color) sensory function and psychomotor performance [16]. Hypertensive Baboons HA, DE, and ME allow comparisons with normotensive baboons (PW, DI, DU) of behavioral impairments produced by antihypertensive drugs across degrees of hypotension produced by the drugs.
Equipment
A shelf for arm support with a 4-inch vertical post at one end to direct the animal's grasp was attached at heart level to the living cage. Blood pressure measurement was accomplished with standard auscultatory equipment consisting of a stethoscope (Tycos, Model No. 5079-28), a child cuff (40.5 X 10.5 cm. W.A. Baum, Inc., Copague. NY) and a bladder (17.5 X 9 cm; W.A. Baum. Inc.) and a mercury (W.A. Baum, Inc., Model No. S96993, "Standby") or aneroid manometer (Carolina Instruments). During BP measurement, the technician read the systolic and diastolic values into a tape recorder, which were later transcribed into a computer.
Blood Pressure Training Procedures
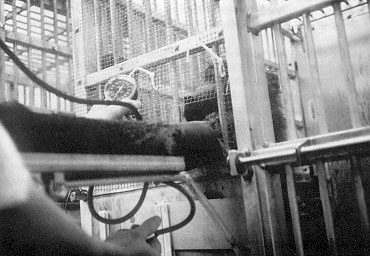
Behavioral conditioning procedures of shaping, fading, and reinforcement were used to train baboons to extend an arm on command onto a shelf at heart level attached to the outside of the living cage and to accept repeated cuff inflations for manual auscultatory BP measurements (Fig. 1). Each measurement included from two to three BP determinations, which were then averaged for both systolic and diastolic values. The scheduling of daily BP measurement was determined by the needs of individual studies and varied between two to three measurements per day. During training, animals were fed their daily ration at 5 p.m. which ensured that the food rewards during BP training were salient reinforcers. Once animals were trained, special scheduling of feeding times was unnecessary, and the amount of food reward during BP measurement was decreased substantially.
The specific training sequences for the BP measurement procedure is described below. After the shelf was attached onto the living cage (Fig. 1), the animal was rewarded with a small piece of food for successfully performing progressively difficult actions: Initially the animal was rewarded for stretching its left arm onto the shelf and accepting food pellets with that hand at the far edge of the shelf; later, the left arm was to be kept extended on the shelf for increasing durations while the animal accepted food pellets with the right hand.
The animal was next rewarded only for grasping the post at the end of the shelf with full arm extension. The next step involved training the animal to allow the extended arm to be stroked with the blood pressure cuff, with the eventual goal of allowing the cuff to be placed briefly around the arm. At this stage, the cuff was opened and closed repeatedly so that the animal habituated to the sound of the velcro fastening and unfastening. While the cuff was in place for extended durations, the animal was rewarded for allowing the stethoscope to touch the arm. With the cuff, stethoscope, and manometer in place (Fig. 1), the cuff was briefly inflated to a small degree while giving frequent food pellets. Improving the quality of reinforcer by switching from food pellets to fresh fruit chunks or continuous apple sauce from an infusion pump into a food nozzle facilitated training. The apple sauce had the added advantage of providing immediate termination of a continuous stream of reinforcement when inappropriate behavior such as arm withdrawal occurred.
The rate and degree of cuff inflation was next progressively increased over successive trials, with termination of reinforcements for arm withdrawal, which occurred frequently at this stage. Once the animal accepted full cuff inflation (to 30 mm Hg above systolic), the cuff was deflated slowly and carefully in order to note the appearance and disappearance of Korotkoff (K) sounds. The animal must be rewarded for sitting through a period of non-reward while the trainer attends to the BP measurement. Again. replacing food pellet delivery with continuously infused dilute apple sauce resulted in more stable behavior with low activity during the measurement procedure. A piece of fresh fruit followed the final cycle of BP measurement.
The trainer should be comfortable and experienced with auscultatory BP measurement, which can be accomplished by enrollment in a brief training course offered by the American Heart Association. The animal training procedure was predictably facilitated by habituating the animals both to the laboratory and to the trainer. Also, several brief training sessions distributed over the day were preferable to one long daily training session.
RESULTS
Resting, Basal Conditions
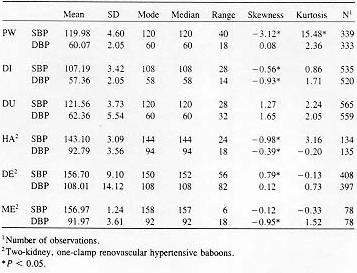
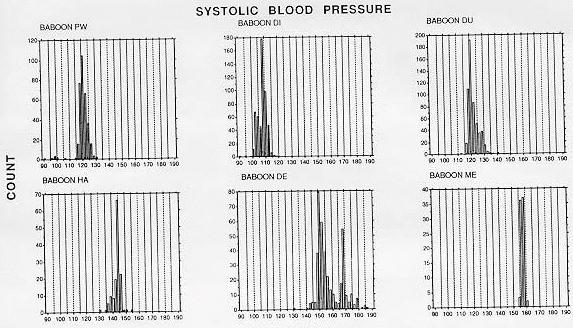
To date, 10 adult male baboons have been trained in the BP measurement procedure. The duration of training until the first systolic and diastolic blood pressure measurements were obtained was an average of 12 weeks (range, 2-36 weeks). We have obtained significant decreases in training time by using the continuous delivery of apple sauce as a reinforcer, the last two baboons were trained in only 3 weeks. SBP and DBP measurements of a fully trained animal require approximately 5 min, which includes set-up of the shelf, food delivery system, and wheeling the manometer in place. Frequency histograms of individual BP determinations obtained after the technicians and the first six baboons were fully trained with the manual auscultatory procedure are shown in Figures 2 (SBP) and 3 (DBP).
The specific training sequence for the BP measurement procedure is described below. After the shelf was attached onto the living cage (Fig. 1), the animal was rewarded with a small pellet of food for successfully performing progressively difficult actions: Initially the animal was rewarded for stretching its left arm onto the shelf and accepting food pellets with that hand at the far edge of the shelf; later, the left arm was to be kept extended on the shelf for increasing durations while the animal accepted food pellets with the right hand.
The animal was next rewarded only for grasping the post at the end of the shelf with full arm extension. The next step involved training the animal to allow the extended arm to be stroked with the blood pressure cuff, with the eventual goal of allowing the cuff to be placed briefly around the arm. At this stage, the cuff was opened and closed repeatedly so that the animal habituated to the sound of the velcro fastening and unfastening. While the Resting SBP pooled across time of day for the normotensive and hypertensive baboons averaged 116.2 (± 7.9 SD) and 152.2 (± 7.9), respectively. DBP averaged 59.9 (± 2.5) and 97.6 (± 9. 0) mm Hg for the normotensive and hypertensive baboons, respectively. Table I lists descriptive statistics for the BP distributions in Figures 2 and 3, with hypothesis testing of skewness and kurtosis measures. With one exception (baboon DE), the medians are within 1-2 mm Hg of the means, although several instances of both positive and negative skewness were found to be significant. Significant kurtosis was found for one animal's SBP (baboon PW). This animal. with a large positive kurtosis value, had a peaked distribution of systolic blood pressure levels.
normatensive and renovascular hypertensive baboons.
Repeated Measurement After Drug Administration
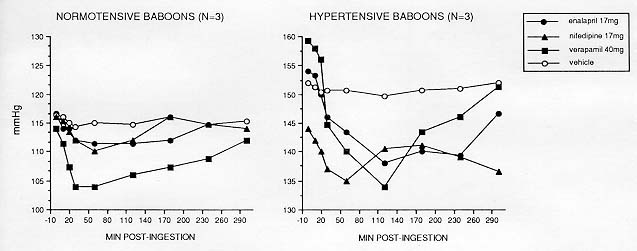
The sensitivity of the manual auscultatory cuff procedure and its feasibility in drug studies with baboons is illustrated in Figure 4 for three antihypertensive drugs encompassing two mechanisms of hypotension: calcium channel blockade (nifedipine and verapamil) and angiotensin converting enzyme inhibition (enalapril). Drug was delivered in powdered form mixed into a small piece of fruit (vehicle). Periodic measurements at 10 min intervals with the technician blind as to drug identity yielded expected immediate decreases in SBP and DBP (DBP data not shown) in relation to vehicle effects. Hourly measurements continued until approximately 4 hr after drug administration, at which time BP returned to predrug baselines.
DISCUSSION
The SBP and DBP levels of the normotensive animals are in close agreement with levels obtained from resting normotensive baboons with blood pressures measured through femoral or carotid artery catheters [5,14,15]. While the distributions were often significantly skewed, systematic positive or negative skewness was not evident. The bimodal shape of renovascular hypertensive baboon DE's frequency histograms resulted from two factors. First, this animal's BP increased by approximately 20 mm Hg during a single 2 week period and restabilized at the higher level. Also, a change in the method of determining DBP was made after approximately 40 determinations: earlier determinations were based on phase V (disappearance of Korotkoff sounds), later ones on phase IV (muffling of sounds). Muffling of sounds occurs at a higher BP level than disappearance of sounds.
Indirect SBPs often have been found in human studies [4] to be lower than direct arterial BP measured through catheters, which has been suggested to result from the direct measurement of BP through the more peripheral radial artery and from incorrect cuff sizes in the indirect method [12]. Indirect BP measurement with an oscillometric method has been validated against simultaneously measured direct radial artery pressure in supine baboons [9]. Automatic oscillometric BP compared favorably with the intra-arterial catheter BP measurements. Average difference between the two methods of determining MAP in anesthetized animals was -1.01 (±5.84) mm Hg. Moreover, the oscillometric readings accurately tracked rapid changes in pressure after i.v. infusion of pressor and depressor drugs.
The indirect measurement of BP in awake animals offers several advantages during chronic experiments. For example, sophisticated and therefore expensive technology such as A-D conversion and associated software are not required. Also, technician time can be devoted to other laboratory tasks after training is completed instead of to the labor-intensive management of catheter-transducer and swivel-harriess systems. The procedure described here uses awake animals, which avoids the effects of ketamine anesthesia on cardiovascular indices such as cardiac output [3].
The potential observer bias in indirect BP determinations when the technician cannot be blinded to experimental procedure can be obviated by use of a random-zero sphygmomanometer (Hawksley Co., Sussex, England). This device randomly varies the amount of mercury in the manometer system before each measurement (range of zero level = 20 mm Hg); the true BP levels are calculated after the reading is taken. Random zero sphygmomanometers have been used as the standard for judging accuracy of, for example, oscillometric machines in human epidemiological studies [171.
The present data demonstrate that acute changes in BP after drug administration can be obtained from the adult male baboon by means of repeated auscultatory measurements. Extended studies of antihypertensive pharmacological agents have also provided evidence for the reliability of these procedures, where. for example, a calcium channel antagonist administered for 21 days produced progressive and weekly BP decreases in awake hypertensive baboons [161. The ultimate advantage of this BP measurement procedure is smoother progress of experiments (at less expense) with animals that are to serve as subjects over many months or years.
MM HG
Class interval, 1 mmHg.
MM HG
ACKNOWLEDGMENTS
This research was supported by USPHS NHLBI grant HL34034. The author thanks K. Craven, M.K. Story, L. Daley, and G. Brinkley for technical support.
REFERENCES
1. Adams MR, Kaplan JR. Manuck SB. Uberseder B. Larkin KT: Persistent sympathetic nervous system arousal associated with tethening in cynomolgus macaques. Lab Anim Sci 38:279-281, 1988.
2. Byrd LD: A tethering system for direct measurement of cardiovascular function in the caged baboon. Am J Physiol 236:H775-779, 1979.
3. Chimoskey JE. Huntsman LL. Gams E, Flanagan WJ: Effect of ketamine on ventricular dynamics of unanesthetized baboons. Cardiovasc Res Center Bull 14:53-57. 1975.
4. Finnie KJ, Watts DG, Armstrong PW: Biases in the measurement of blood pressure. Crit Care Med 12:965-968, 1984.
5. Harris AH, Turkkan JS: Performance characteristics of conditioned blood pressure elevations in the baboon. Biofeed Self-Regul 6:11-24,1981.
6. Krausman DT: Methods and procedures for monitoring and recording blood pressure. Am Psychol 30:285-294, 1975.
7. Lukas SE, Griffiths RR, Bradford LD, Brady JV. Daley L, Delorenzo R: A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol Biochern Behav 17:823- 829, 1982.
8. McGill HC Jr. Carey KD, McMahan CA, Martinez YM, Cooper TE, Mott GE, Schwartz CJ: Effects of two forms of hypertension on atherosclerosis in the hyperlipidemic baboon. Arteriosclerosis 5:481-493. 1985.
9. Mitchell DS, Peel HH, Wigodsky HS, Morris M: Noninvasive oscillometric measurement of blood pressure in baboons (Papio cynocephalus). Lab Anim Sci 30:666-672, 1980.
10. Mitchell DS, Graham JR, Castracane VD: Operant elevation of blood pressure in unrestrained olive baboons (Papio cynocephalus anubis). Am J Primatol 3:229-238, 1982.
11. Patrick T, Vatner SF: Instrumentation techniques for cardiovascular research in conscious animals. In: Linden RJ (ed): "Techniques in Life Science: Cardiovascular Physiology." Shannon, Ireland: Elsevier Scientific Publishers, 1984, 1-22.
12. Rebenson-Piano M, Holm K. Powers M: An examination of the differences that occur between direct and indirect blood pressure measurement. Heart Lung 16:285-294, 190.
13. Report of Task Group on Restrained Animals Considerations. In: Herd JA, Gotto AM. Kaufmann PG. Weiss SM (eds): "Cardiovascular Instrumentation Proceedings of the Working Conference on Applicability of New Technology in Biobehavioral Research." NIH Publications No. 84-1654. 1984, 291-296.
14. Turkkan JS, Ham's AH: Shaping blood pressure elevations: An examination of acquisition. Behav Anal Lett 1:97-106, 1981.
15. Turkkan JS. Harris AH: Hyperdipsia in the baboon during operant conditioning of blood pressure elevations. Behav Neurosci 98:631-639, 1984.
16. Turkkan JS. Hienz RD: Behavioral performance effects of nifedipine in normotensive and renovascular hypertensive baboons. Psychopharmacology (in press), 1989.
17. Whelton PK. Thompson SG. Barnes GR, Miall WE: Evaluation of the Vita-Stat automatic blood pressure recorder: A comparison with the random-zero sphygmomanometer. Am J Epiderniol 117:46- 54, 1983.
This article originally appeared in the Journal of Medical Primatology 19, 455-466 (1990).
Reprinted with permission of the Editor.