Bernadette M. Marriott,1 Robert W. Marriott, Jr.,2 Jean Norris,1 Deborah Lee1
1 Goucher College, Towson, MD
2 Systems, Environment and Behavior, Incorporated, Baltimore, MD, U.S.A.
Accepted for publication August 23, 1993.
Abstract
A semi-natural habitat that was designed to house a social group of squirrel monkeys (Saimiri sciureus sciureus) at Goucher College, in Maryland is described. The design could be readily adapted for use with other small primate species.
Key words: naturalistic group enclosure - squirrel monkeys - design
Introduction
Recent concern regarding the impact of captive environment on the psychological well-being of nonhuman primates has stimulated a large number of experimental studies in which the behavior, activity, stimulus reactivity, or physiology [8,13,16] of monkeys has been reported. These studies have measured responses to changes in cage or enclosure size [9,18,29], novel objects [5,8,14,17], feeding devices [1,2,7,15], cage furniture [22], etc. The results have been variable and often conflicting [cf, 9,24]. While objective data continue to be collected in response to such changes in cage size, most reports clearly indicate that access to a social partner can increase the frequency of species-typical behavior and may reduce stereotypical patterns [20-23]. In addition, several studies [ 11,30-32] have shown that confining formerly free-ranging rhesus monkeys and patas monkeys to small corrals led to increased joint mobility, the reversibility of which was shown to be dependent on age at confinement.
With the advent of recent regulations regarding housing of nonhuman primates, most laboratory personnel are currently considering myriad alternatives available for their facilities. Research has shown that placing formerly singly housed nonhuman primates in groups of unrelated individuals can be done successfully if the introductions are carefully planned and the stress responses of subordinates are monitored [20,21,27]. For treatment and experimental purposes, however, it is often desirable to have rapid access to individual monkeys without using nets, poles, or other stressful means of capture.
The purpose of this brief report is to describe a facility that was inexpensively developed to house a social group of New World primates for teaching and research purposes at a small college in the northeastern United States. A feeding/capture cage and remote guillotine door arrangement permitted rapid access to individual animals without the stress of netting or hand capture. The ability to partition the facility in half further permitted temporary isolation of ill or injured animals without drastic environmental change and allowed increased accessibility. This facility was developed on the foundation and frame of a former greenhouse. A similar arrangement could be made, however, by removing cage racks and remodeling an existing indoor animal room.
This facility was designed and maintained to exceed the existing regulations for primate housing under the guidance of a veterinarian who was board-certified in Laboratory Animal Medicine. It was registered with, and regularly inspected by, veterinarians from the United States Department of Agriculture (USDA) who recommended the facility as a model of its type. Although the concept of an indoor naturalistic enclosure for primates is not new [17,19], attention to the details of heating, cooling, cleaning, and access during the design process resulted in a facility that was inexpensive to construct and maintain. Such a facility was thus suitable for a small institution and provided the advantages of social housing for the animals and observers while retaining the potential for rapid access to individual monkeys.
The design described below was developed based on the knowledge (circa 1981) of squirrel monkey social behavior, social spacing, foraging, and locomotor style. Care was taken to develop a complex arboreal pathway that included links at all levels in the vertical space of the enclosure. Sizes and textures of substrates were varied, with flat areas and freely mobile items provided for use as play surfaces/ structures.
Facility design
Habitat description and use
The semi-natural Primate Facility at Goucher College consisted of the "Habitat," which housed the animals and an attached space that functioned as an animal treatment area and observation room. (See floor plan in Fig. 1.) The facility was in operation from January, 1982 through July, 1985, when it was closed because the animal colony moved with the first author to another institution.
The entire facility was located within a cinderblock building that was built using the foundation and supports of an existing greenhouse. Systems for environmental control were added to the existing water and electrical circuits, the glass was removed, the concrete walls were extended, and a solid roof was constructed. The galvanized 2.5 cm X 5.08 cm wire ceiling and non-block interior walls of the Habitat area were entirely covered with a thin coat of smooth cement to facilitate cleaning and reduce deterioration. All surfaces were then painted with a nontoxic, high-gloss, waterproof paint to provide an impervious surface. If built today, replacement of the concrete with one of the new, impervious plastic wallboards approved by the USDA for use in animal facilities, such as LascoBoard (Lasco Panel Products, Orlando, FL), would have reduced construction costs further.
The Habitat was 7.5 in long X 7.0 in wide X 2.6 in high (interior dimensions). The floor consisted of a 15 cm layer of gravel on an earth base, which allowed percolation of waste matter. A mesh ceiling separated the animals from the environmental control systems.
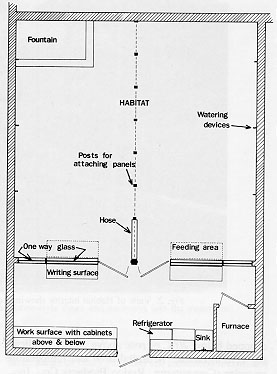
Six large skylights in the roof provided natural lighting, augmented by eight automatically timed pairs of fluorescent lights. Below the lights were a series of misters (J.A. Nearing Co., Inc., Laurel, MD) that produced a fog-like rain. The misters were automatically timed to spray the area inside the Habitat twice a day for a four minute duration. This fine-mist system maintained a relative humidity of 72-75%, simulating the relative humidity found in South American forests. During the summer months, misting was set to occur more frequently to assist with maintaining a constant, year round temperature.
A temperature range of 21º-33ºC was primarily maintained through thermostatically controlled heating and ventilation systems. The heat source was a gas-fired, forced-air system. Two fan-forced electric heaters provided a backup for system failure. A variable-speed, thermostatically controlled ventilation fan in the eave of the roof cooled the Habitat. Fan speed was lowered in winter to conserve energy while providing the required air exchange rate and was increased in the summer to provide cooling. There was a separate, variable-speed circulating fan in the Habitat. Alarm lights located on the outside of the building were illuminated when the temperature within the Habitat dropped below 20ºC or rose above 34.5ºC, indicating system failure. A digital time and temperature recorder (Computemp, Rodco Products Co., Inc., Columbus, NE) was used to record the ambient temperature and daily maxima and minima both inside the Habitat and in the treatment room. Water was available to the animals ad libitum from the daily rain, from a continuously running fieldstone foundation located in one corner of the Habitat; and from five automatic watering devices (Adjustable Flow Laboratory Animal Drinking Valves, Edstrom Industries, Inc., Waterford, WI) located near the Habitat floor. Potted plants (Ficus benjamina and F. pumilla)were embedded in the gravel floor and fountain area. Interconnecting pieces of bamboo and tree limbs suspected from the ceiling by ropes and supported by vertical, upright tree trunks provided a multilevel, complex pathway for locomotion. Freely hanging ropes as well as tree stumps set in the floor also provided areas for the animals to run, jump, climb, and rest. (see Fig. 2.)

Access to the mechanical systems on the Habitat ceiling was through panels in the treatment area. When more extensive repairs were required, the Habitat was partitioned into two sections through the use of opaque, corrugated plastic, wood-framed dividers that locked into place along a center row of support columns. The entire group of animals could be moved to one side of the Habitat while work was carried out on the other half. This feature also enabled the maintenance of two separate columns or segregation of ill animals, if needed.
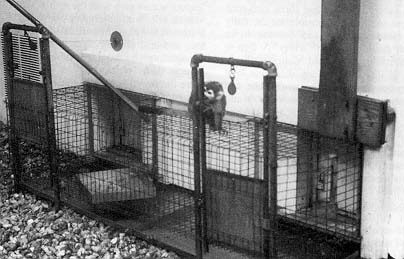
The wall separating the Habitat from the treatment area contained two large (183 cm X 127 cm) and two small (123 cm X 50 cm) one-way vision windows that allowed unobtrusive observation of the animals' activities. Two painted metal doors, one on either side of the center columns, provided access to each half of the Habitat. Each door had a 46 cm X 74 cm one-way vision window so that an almost totally uninterrupted view of the Habitat was available to the observer. Two galvanized (I inch square) wire mesh tunnels were located inside the Habitat directly underneath the observation windows (see Fig. 3). The tunnels were modeled after an interconnecting system previously used with colony rack cages [25]. Each tunnel was "C"-shaped and 152.4 cm long, with 45.72 cm extensions at each end that attached to stainless steel doors (46 cm X 30 cm) in the wall that separated the Habitat from the treatment room. The monkeys could enter the tunnels only when guillotine- style, stainless steel doors that were located in the tunnel were raised. The doors could be raised and lowered from the treatment area using a pulley system from within the treatment area. Food was prepared in the treatment area and placed twice a day in the tunnels. The guillotine doors were then raised to provide entry for the animals. The monkeys received food only in the tunnels. In this way, they became habituated to entering the tunnels and were easily captured, when necessary, by placing favored foods in the tunnel, then remotely lowering the guillotine door when the desired monkey entered the tunnel. The monkeys were easily enticed to enter transfer cages through the doors to the treatment area. This method of capture eliminated the substantial stress associated with netting or hand capturing.
The tunnels also were used for gradual introduction or reintroduction of monkeys into the colony. When a newly acquired monkey or one that had been isolated due to injury or illness was ready to join the group, one of the wire mesh feeding tunnels was closed, and the remaining animals were fed in the other tunnel. The monkey to be introduced was placed in a closed transfer cage next to the open outer door of the tunnel for two days. In this way, visual, auditory, and olfactory contact was established or reestablished between the novel individual and the group without the possibility of physical contact. On days three and four, the door to the transfer cage was opened, giving the new animal the option of initiating physical contact. On day five, the novel animal was placed in the closed tunnel. On day six, the guillotine door was raised and the introduction was complete. Twelve of the animals in the original group were acquired from other institutions where they had been singly housed. Upon arrival, all of these individuals were missing large areas of body fur, and six exhibited locomotor stereotypies. All were gradually introduced into the social group using the mesh tunnels in the Habitat area. By using this procedure, there was no contact aggression directed toward the newly introduced monkeys. After introduction, all animals regained full body fur, gained body weight, and no longer exhibited locomotor stereotypies, with the exception of one hand-reared juvenile female who continues to pace during stressful situations.
Treatment/observation room
The treatment area was 7.0 m wide X 2.4 m deep. A refrigerator, large, stainless steel sink, and wall-mounted cabinets provided storage for supplies and cleaning equipment. Caging for sick or injured animals fit into recessed areas along the end walls. All thermostats and timers were located in this room, eliminating the need for entering the Habitat except for periodic cleaning. Fold-down tables, attached below the large observation windows, provided a work area for observers. Strip electrical outlets were located above all work surfaces.
Maintenance and animal health
The Primate Facility was maintained by a rotating staff of four part-time employees. This included a colony manager who supervised the animal care, monitored the general health of the animals, maintained supplies, and coordinated the timing of student research projects. The other three part-time employees were students responsible for the daily feeding and cleaning duties and for the twice weekly general cleaning of the facility. A sign-in book was maintained to record visitor/staff entrances to the facility. A daily log sheet was kept by the employees describing the work done and status of the animals and facility. Checklists on animal health status, maintenance duties, and standard feeding procedures were also maintained. Face masks, gloves, and lab coats were worn by all people who entered the facility.
The twice weekly cleaning was performed by a pair of students wearing protective clothing, boots, hats, and face masks. The students entered the Habitat and collected uneaten food and plant debris from the floor. All non-structural substrates (bamboo poles, jute rope, tree trunks, plants) were carefully examined and adjusted or replaced as needed. The walls, windows, and substrates were then scrubbed with a mild soap and rinsed. Next, the gravel floor was raked to break up and distribute fecal matter. Finally, the floor was rinsed with a hose to accelerate the percolation of waste through the gravel floor. The treatment/ observation room was then thoroughly cleaned. Each twice weekly cleaning took approximately one hour to complete. Student caretakers were issued protective jumpsuit-style uniforms that were collected with all lab coats and cleaned weekly by a commercial laundry that serviced the college. Periodic pest control services were performed as part of the overall contract for the college, with review by the attending veterinarian.
The Primate Facility at Goucher College was primarily used for teaching and observation of primate social behavior. The staff had no direct contact with the monkeys aside from twice weekly cleaning in the Habitat. The animals were visually checked daily for health status by observing each animal for one minute through the one-way glass.
When the facility was in operation from January 1982 through July 1985, no animals became ill or died due to illness. One animal died in the first 24 hours after introduction into the facility as a result of a head injury during a fall. Before introduction into the Habitat, the monkeys had been housed as a social group in a series of large cages that were interconnected by mesh tunnels [241. This caging configuration allowed social contact, short-distance running and jumping, swinging, and climbing. For the first four days after introduction into the Habitat, the monkeys exhibited striking imbalance when walking on all substrates with a diameter of 3 cm or less. All individuals avoided walking on horizontally suspended ropes for the first week to 10 days. They also appeared to misjudge distances when leaping across gaps with the result that several individuals fell, with one injury-an adult female that fractured her skull and died. Most animals would leap and land slightly short of their goal but close enough to grab onto a tree limb extension or rope. Seeing these problems, we immediately added more linking substrates so that there were very few places where an animal would need to leap to complete a pathway. Research that has specifically addressed the physical effects of housing animals in cages and small enclosures is limited [11,30-32], but it was clear to us that there had been a change not only in muscle tone but also in distance perception/motor responsiveness as a result of caging these animals, even in spacious conditions. Whenever caged monkeys are released into larger enclosures, we therefore recommend careful observation for individual problems due to change in muscle tone or perception that may affect movement capabilities.
Aside from yearly physical examinations, there were only three occasions in the 3½ years that required animal removal and veterinary care: one animal was treated for an incomplete abortion [28] and there were two instances of bite wounds to the same pubertal male that required sutures. Nine live- born infants, one still-born infant, and one abortion were produced out of a total of 15 female reproductive years from 1982 through July 1985. There were no infant deaths.
Discussion
A semi-natural style enclosure provides a number of advantages over cages for housing nonhuman primates. Animals maintained in this semi-natural Habitat at Goucher College were healthy, and none of the animals exhibited locomotor stereotypies. This facility was easier and more economical to maintain than traditional caging. Access to the animals was only slightly diminished. Using the previously described capture technique, animals were readily caught. Repeated, frequent capture of animals for biomedical sampling is possible with this facility design because animals could be trained for such procedures with little disruption of the social dynamics of the group [3,4,6,10]. In this facility, the animals displayed diurnal activity rhythms congruent with free-ranging squirrel monkeys and a seasonal birth peak [Marriott and Meyers, unpublished manuscript]. Construction of the facility was inexpensive because it utilized an existing steel support frame and foundation wall, but any large room could be converted to a similar facility. It proved to be an excellent teaching space for introductory courses in animal behavior and specific studies of squirrel monkey social behavior, as well as animal handling and care.
Since both Old World and New World nonhuman primates can be trained to enter catching cages, social housing of monkeys in spaces that allow expression of normal locomotor patterns for the species should be considered as viable alternatives for behavioral and biomedical research colonies. Particularly for smaller primate species, the need for frequent handling for biomedical protocols should not preclude group housing. If rooms that are presently used to hold cage racks in an institution are converted to semi-natural style enclosures, more colonies would be able to set aside a percentage of their animals for breeding.
Enriched environments may be particularly necessary for expression of more species-typical repertoires and behavioral frequencies in New World primates [26]. Maintenance of diversity and frequency of behaviors is important not only in teaching settings, but also in biomedical research design where frequency of behavioral units is the dependent variable. As recently discussed [4,12], social and physical enhancement of captive settings for nonhuman primates can benefit from data on the same species in natural environments. Using field data as a guide, designs for primate enclosures can maximize the available space for the animals through placement of perches, pathways, and cage furniture in locations that allow species-typical behavior patterns to mimic those in free-ranging conditions. Semi-natural habitats [cf. 17] with appropriate capture chutes should thus be considered as viable alternatives to caging for most experimental paradigms.
Acknowledgments
We would like to thank M.M. Swindle, DVM; Nelson Garnet, DVM; Michele Cichon; Sue Eller; Andrea O'Mara; Duffy Bauer; Katie Groner; and Pam Hamblett for their careful and attentive care of the monkeys. The help of Paul Petrick, Richard A. MacIntyre, the staffs of the Goucher College Physical Plant, and the Security Department were essential to the safety of both the animals and staff. In particular, we are grateful to the late James Billet, Dean; Rhoda Dorsey, President; Jean Bradford; Susan Cowles; Barbara Long; and Richard Pringle, Goucher College, for their encouragement and financial support. We would like to acknowledge Dr. E.W. Menzel, whose greenhouse facility for housing marmosets at the State University of New York at Stony Brook was the inspiration for this design.
References
1. BAYNE K, MAINZER H, DEXTER S, CAMPBELL G, YAMADA F, Suomi S: The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/ grooming board. Am J Primatol 23:23-36, 1991.
2. BECKLEY S, NOVAK M: An examination of various foraging components and their suitability as enrichment tools for captively-housed primates. Am J Primatol (Suppl 1): 37-44, 1989.
3. BERNSTEIN IS: Research in a breeding colony. In Buckelow WR (ed): "Nonhuman Primate Models for Human Diseases." Boca Raton: CRC Press, 1983.
4. BERNSTEIN IS: Breeding colonies and psychological well-being. Am J Primatol (Suppl 1):31-36, 1989.
5. BRENT L, LEE DR, EICHBERG JW: Evaluation of two environmental enrichment devices for singly caged chimpanzees (Pan troglodytes). Am J Primatol (Suppl 1):65-70, 1989.
6. BUNYAK SC, HARVEY NC, RHINE RJ, WILSON MI: Brief Report: Venipuncture and vaginal swabbing in an enclosure occupied by a mixed-sex group of stumptailed macaques (Macaca arctoides). Am J Primatol 2:201-204, 1982.
7. BYRNE GD, Suomi S: Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. Am J Primatol 23:141-152, 1991.
8. CLARKE MR, KORITNIK DR, MARTIN LN, BASKIN GB: Cage enrichment, physiology, and behavior in nursery-reared rhesus monkeys. Am J Primatol (Suppl 1):53-58, 1989.
9. ERWIN J, SACKETT GP: Effects of management methods, social organization, and physical space on primate behavior and health. Am J Primatol 20:23-30, 1990.
10. GORDON RP, ROSE RM, BERNSTEIN IS: Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): A three year study. Horm Behav 7:229-243, 1976.
11. KESSLER MJ, TURNQUIST JE, PRITZKER KPH, LONDON WT: Reduction of passive extension and radiographic evidence of degenerative knee joint diseases in cage-raised and free- ranging aged rhesus monkeys (Macaca mulatta). J Med Primatol 15:1-9, 1986.
12. LINDBURG DG: Ecological requirements of macaques. Lab Anim Sci 41:315-322, 1991.
13. LINE SW, MORGAN KN, MARKOWITZ H, STRONG S: Influence of cage size on heart rate and behavior in rhesus monkeys. Am J Vet Res 50:1523-1526, 1989.
14. LINE SW, MORGAN KN: The effects of two novel objects on the behavior of singly-caged adult rhesus monkeys. Lab Anim Sci 41:365-369, 1991.
15. MAKI A, ALFORD PL, BLOOMSMITH MA, FRANKLIN J: Food puzzle device simulating termite fishing for captive chimpanzees (Pan troglodytes). Am J Primatol (Suppl 1): 71-78, 1989.
16. MENDOZA SP: Sociophysiology of well-being in nonhuman primates. Lab Anim Sci 41:344-349, 1991.
17. MENZEL EW, MENZEL CR: Cognitive, developmental and social aspects of responsiveness to novel objects in a family group of marmosets (Saguinus fuscicollis). Behav 70:251-274, 1979.
18. NASH LT, CHILTON SM: Space or novelty?: Effects of altered cage size on Galago behavior. Am J Primatol 10: 37-49, 1986.
19. PRICE EC, McGREW WC: Cotton-top tamarins (Saguinus (o.) oedipus) in a Semi- naturalistic captive colony. Am J Primatol 20:1-12, 1990.
20. REINHART V: Preliminary comments on pairing unfamiliar adult male rhesus monkeys for the purpose of environmental enrichment. Lab Prim Newsl 27:1-3, 1988.
21. REINHART V: Re-pairing of caged rhesus monkeys. Lab Prim Newsl 28:19, 1989.
22. REINHART V: Time budget of caged rhesus monkeys exposed to a companion, a PVC perch, and a piece of wood for an extended time. Am J Primatol 20:51-56, 1990.
23. REINHART V, HOUSER D, EISELE S, COWLEY D, VERTEIN R: Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. Am J Primatol 14:135-140, 1988.
24. ROBERTS JA: Environmental enrichment, providing psychological well-being for people and primates. Am J Primatol (Suppl 1):25-30, 1989.
25. SALZEN EA, MARRIOTT BM: Technical note: a primatrail or an inexpensive cage expansion for group housing small primates. J Med Primatol 20:94-96, 1991.
26. SCHOENFELD D: Effects of environmental impoverishment on the social behavior of marmosets (Callithrix jacchus). Am J Primatol (Suppl 1):45-51, 1989.
27. SNYDER DR, PILBEAM DR: The need for field-laboratory facilities for primate research. J Med Primatol 2:257-266, 1973.
28. SWINDLE MM, MARRIOTT BM, FRANKS AA: Incomplete abortion and hydrometriosis in a squirrel monkey (Saimiri sciureus). Lab Anim Sci 34:290-292, 1984.
29. TARANTINO SJ: Effect of cage confinement on social behavior in squirrel monkeys. Psychon Sci 20:294-29. 1970.
30. TURNQUIST JE: Influence of age, sex and caging on joint mobility in the patas monkey (Erythrocebus patas). Am Phys Anthropol 61:211-220, 1983.
31. TURNQUIST JE: Passive joint mobility in patas monkeys (Erythrocebus patas): rehabilitation of caged animals after release into a free-ranging environment. Am J Phys Anthropol 67:1-5, 1985.
32. TURNQUIST JE: Cages, corrals, and natural habitat enclosures: their effect on post-cranial morphology and joint mobility in rhesus monkeys. Am J Phys Anthropol 75:281, 1988.
This article originally appeared in the Journal of Medical Primatology 22, 348-354 (1993).
Reprinted with permission of the Editor.