V Reinhardt, C Liss and C Stevens
Animal Welfare Institute, P0 Box 3650, Washington, DC 20007, USA
Abstract
Cage space requirements for non-human primates in the United States of America are less than those in European countries. Studies in support of the assumption that the US legal minimum cage size provides adequate space have limited value because they only tested cages without structural enhancement. It is not surprising that non-human primates cannot be animated to be more active or to behave in more species-typical manners by only providing them with extra barren space. Explicitly stipulating that all cages have to be equipped with properly installed, elevated structures appropriate to each species and age category would make the US standards more adequate. Such structures would no longer restrict the caged primate to an unnatural, permanent terrestrial lifestyle but would allow the animal to make use of the arboreal, 'safe' dimension to which she/he is biologically adapted. Minimal height requirements will have to be upgraded in the US to accommodate these ethological considerations.
Keywords: animal welfare, non-human primates, quality of cage space, regulations
Published in Animal Welfare 5:(4), 361-372 (1996)
© 1996 Universities Federation for Animal Welfare
This article has been reproduced with the permission of the publisher.
Introduction
Primatologists agree that adequate cage space is a key factor for a monkey's well-being. Bayne (1989) asked 56 investigators of 10 laboratories in the United States what they thought could be done to improve their animals' well-being. The most frequent recommendation was for larger cages (cf. National Institutes of Health 1991). This finding is not surprising when considering the fact that cage dimension requirements are less than standard in the United States of America in comparison with European countries (Table 1).
Despite this apparent awareness that non-human primates in the US deserve larger cages than those currently used, there is a strong reluctance to increase cage sizes because of the perceived costs (Bowden 1988; Holden 1988; Line et al 1989a, b; 1991; Mason 1989; Woolverton et al 1989; Erwin 1991; Wolfle 1991; American Medical Association 1992; Crockett 1993), and because of recent studies which support the status quo by demonstrating that the quantity of cage space is not an important factor in the promotion of primate well-being. The present report reviews these studies and evaluates the usefulness of their methodologies in determining species-adequate cage space standards for non-human primates.
Table 1: Minimal cage space requirements for the most common laboratory nonhuman primates in Europe* (European Economic Community Council 1986) and in the USA (National Institutes of Health 1985; United States Department of Agriculture 1991).
| Floor area (m²) | Height (m) | |
|---|---|---|
| Female macaques (5-7kg) |
0.70 (Europe) |
0.85 (Europe) |
| Male macaques (10-15kg) |
1.10 (Europe) |
1.25 (Europe) |
| Baboons (15-25kg) |
1.50 (Europe) |
1.25 (Europe) |
* Space requirements of some countries, such as Great Britain (Home Office 1989) and Switzerland (Der Schweizerische Bundesrat 1981) exceed the EEC standards.
Activity
Line et al (1989a, b; 1990a; 1991) monitored locomotor activity in 10, and 6 adult female rhesus macaques (Macaca mulatta) each housed singly for one week at a time in three over-legal size, barren cages (0.40m2x0.81m; 0.57m2x0.81m; 0.62m2x1.l0m). The animals showed no significant changes in activity as a function of cage dimension. The authors inferred from this that changes in the current cage sizes will not improve the animals' well-being in any measurable way (Line et al 1989a; 1990a).
It may seem a matter of common sense that non-human primates housed in larger cages would be better off than those housed in smaller ones (Chamove 1989; Chamove & Anderson 1989; Line et al 1989a), yet it is hard to imagine that any animal can benefit from space that is not structured. Space is not enough; there must be something in the space (Wilson 1982) to make it useful. Even an over-legal size cage will not make a monkey climb, perch, and explore if there is nothing to climb and perch on, and if there is nothing to be explored. It would be unjustified to classify such a barren cage as unnecessarily large because the occupant fails to be particularly active.
Brent (1992) observed four individually housed chimpanzees (Pan troglodytes) in different-sized cages that were not barren but equipped with structuring elements. Under this condition, significantly more activity was noted in larger than in smaller cages. This is congruent with similar findings by Daschbach et al (1983) and Kaplan and Lobao (1991), indicating that the well-being of a primate, as measured by his/her activity, is primarily dependent on the quality rather than on the quantity of space, since space per se has no stimulatory value.
Non-human primates are characterized by major anatomical and behavioural adaptations to a three-dimensional arboreal environment (Reynolds & Reynolds 1965; Schultz 1969; Rose 1974; Estrada 1989; Martin 1990). This suggests that they have a particular need for structured vertical space. Both in the wild and in captivity most species show vertical flight reactions (Jay 1965; Crockett & Wilson 1980; Estrada 1989; Taff & Dolhinow 1989; Burt & Plant 1990; Chopra et al 1992; Watson & Shively 1996) and spend a major portion of their time off the ground on trees, cliffs, 'koppes' or artificial structures (Bernstein & Mason 1963; Rosenblum et al 1964; Goodall 1965; Simonds 1965; Menzel 1967; Traylor-Holzer & Fritz 1985; Watson 1991; Chopra et al 1992). In the wild, the presence of large trees may be the only limitation for the distribution of primates (DeVore & Hall 1965; Simonds 1965; Washburn & Hamburg 1965). In captivity most primates will locate themselves as high as possible rather than on or close to the ground (Williams & Abee 1985; Salzen 1989; Field et al 1992; Reinhardt 1992; O'Neill-Wagner 1994; Weed et al 1995; KerI & Rothe 1996; Watson & Shively 1996). European regulations therefore stipulate that perches be installed (Der Schweizerische Bundesrat 1981; European Economic Community Council 1986; Home Office 1989). They make the vertical dimension of the cage accessible for specialized, i.e. arboreal locomotor patterns (National Institutes of Health 1985; United States Department of Agriculture 1991) such as climbing, leaping, balancing, and for specialized comfort behaviours such as perching, retreating and looking-out (Reinhardt et al 1987a; Reinhardt 1989; Watson 1991). European cage height requirements exceed those of the USA mainly because extra space is needed for the proper placement of perches (cf. Table 1).
Housing non-human primates in empty cages that are not organized to suit their species-specific needs for arboreal behaviour, is as inappropriate as housing them singly without means to show social behaviour. Fundamental requirements for the expression of 'behavioural needs' (European Economic Community Council 1986; Line 1987; United States Department of Agriculture 1991; Poole 1992; International Primatological Society 1993) are not met in either circumstance. US rules take the animals' needs for the expression of social behaviours adequately into account but fail to address those for the expression of arboreal behaviours (United States Department of Agriculture 1991).
Stress
Crockett et al (1993a) assessed urinary cortisol excretion in 10 adult female and 10 adult male long-tailed macaques (Macaca fascicularis) housed individually in five different-sized single cages. Floor areas of two cages were 7 per cent and 40 per cent larger than legal size, three cages were 23 per cent, 55 per cent and 77-83 per cent smaller than legal size. There was a tendency for cortisol levels to increase as cage size area decreased, but statistical analysis led to the conclusion that cortisol was unrelated to cage size (Crockett et al 1993a). Replication of the study with eight female pig-tailed macaques (Macaca nemestrina) yielded the same results (Crockett et all 993b). The authors inferred that the impact of a wide range of different cage sizes on the well-being of the monkeys was small and that the legal minimum cage size did provide adequate space (Crockett & Bowden 1994).
Cortisol is apparently not a good indicator for determining adequate cage space appropriations for non-human primates. The smallest cages examined were so small that they allowed almost no unhindered movements (0.05m2x0.36m, 0.10m2x0.43m; cf. Crockett & Bowden 1994, Figure 2, right). Yet, being squeezed in these tiny cages, the animals showed no significant cortisol elevation. Increasing the dimensions of such an 'unrealistically small' (Crockett et al 1993a) cage intrinsically promotes the well-being of the confined subject by easing the spatial restrictions of its species-characteristic postures and movements. It would be absurd to classify a cage as species-adequate because the occupant fails to show a particular physiological response but does not have enough room to stand upright or turn around. The problems associated with the interpretation of cortisol data in relation to housing changes, have also been encountered when comparing data of single-housed versus compatible pair-housed social primates (Reinhardt et al 1991; Schapiro et al 1993; Crockett et al 1994). Cortisol levels did not differ within any of these studies, indicating that neither housing condition is a special source of stress. This, however, would not allow the inference that both conditions are species-adequate. Needless to say, single-housing is not adequate for any social species.
The sensitivity of caged non-human primates to insufficient quantity of space was drastically demonstrated in a study conducted by Boot et al (1985). The authors evaluated reproductive performance in single-caged multigravid long-tailed macaques kept in two different-sized cages. Pregnancy loss was 31 per cent in 67 females housed in sublegal-sized cages (0.20m2x0.60m) versus 8 per cent in 37 females housed in legal-sized cages (0.49m2x1.00m). The difference was significant, suggesting that being forced to live in the very small cages was a distressing experience for the subjects.
Behavioural disorders
Crockett and Bowden (1994) examined the effect of cage space on the expression of behavioural disorders. The above long-tailed and pig-tailed macaques were videotaped in a familiar environment (Crockett et al 1993a) when being single-housed for two weeks, each in five different-sized barren cages (largest cage: 0.61m2x0.84m; smallest cage: 0.05m2x0.36m). Smaller cages failed to trigger an increase in abnormal behaviours. These findings are in line with those of Bayne and McCully (1989) and Line et al (1989a; 1990a; 1991): six and ten adult single-housed rhesus macaques were tested in small and moderately enlarged barren cages (0.40m2 versus 0.57m2 versus 0.63m2). No reduction in stereotypical behaviours was seen in the larger cages. It was concluded that increasing cage size does not lead to improvements in well-being (Crockett & Bowden 1994).
It would be surprising if intelligent animals such as primates could be cured from ingrained behavioural disorders by simply being provided with more empty space. Even a manyfold enlargement of unstructured cage space will not change this circumstance (Goosen 1988). The situation is different when increased space also implies more complexity. Leu et a! (1993), for example, noted a significant decrease in stereotypical activities in 20 single-housed long-tailed macaques transferred daily from barren home-cages to large multi-compartmentalized cages. Exploring the various compartments of the larger cage probably distracted the animals sufficiently to override the urge for stereotyped movements.
The predominant importance of the quality rather than quantity of cage space for the behavioural health of non-human primates is underscored by the fact that 'pathologic behaviour' (Brent et al 1989), especially stereotypies, is ameliorated or even eradicated when no extra space is provided, but suitable stimuli added to counteract 'boredom' (Whitney & Wickings 1987; Vandenbergh 1989; Wemelsfelder 1994) in a hitherto barren cage environment (Reinhardt et al 1987b; Goosen 1988; Brent et al 1989; Line et a! 1989c, 1990b; Meunier et a! 1989; Weld et al 1989; Weld & Erwin 1990; Bayne et al 1991; Lam et al 1991; Bayne et al; 1992a, b; Nadler et al 1992; Perkins et al; 1992; Eaton et al 1993; Phillippi-Falkenstein 1993; Shimoji et al 1993; Boinski et al 1994; Brent & Long 1995).
While behavioural disorders are not significantly affected by the actual cage size, sporadic disorders may be triggered in an animal when the cage becomes too small for normal 'distancing responses' (Hediger 1955) during an alarming situation. The confined subject is quasi cornered in such a circumstance and experiences higher tension (Berkson et al 1963) than in a large cage with adequate options for flight. Draper and Bernstein (1963) noticed that rhesus macaques resort to stereotyped activities in an unfamiliar, potentially alarming environment when being confined in small cages (0.64m2x0.80m) but not when being confined in much larger cages (3.84m2x1.60m) with enough space for escape. The authors hypothesized that the stereotyped movements served the subjects as substitute actions for inhibited flight responses to the fear-inducing, unfamiliar environment. This inference is in line with the commonly made observation that caged primates afflicted with gross behavioural pathologies such as self-biting or self-clasping, predictably display these reactions when being approached or looked at by a fear-provoking person (Reinhardt 1995, Slides 34-36; cf. Berkson 1968; Fittioghoff et al 1974; Fritz et al 1992).
Discussion
Assessing the quantity of cage space on the well-being of primates has limited value because the usefulness of space depends on its quality rather than on its quantity (cf. Erwin & Deni 1979; Wilson 1982; Whitney & Wickings 1987; Chamove 1989; Line et al 1989b; Anderson & Visalberghi 1990; Schapiro et al 1991; Ruempler 1992; International Primatological Society 1993; Leu et al 1993; Benn 1995). Having no stimulatory value, space alone does not enhance an animal's environment.
There is professional agreement that adequate cage size is best determined by behaviour-based criteria. These criteria set the standards for the quality of the cage volume, which in turn determines the quantity of space that is required to meet basic behavioural needs of the caged subject. The cage should not only provide enough room for normal posture, postural changes and movements on the ground (cf. National Institutes of Health 1985; United States Department of Agriculture 1991), but its space must be sufficiently usable and complex to allow for species-specific locomotor and posture characteristics above the ground (International Primatological Society 1993). Vertical climbing surfaces and perches are therefore recommended (International Primatological Society 1993) as a means of making the space of the cage usable for the expression of arboreal behaviours. Furthermore, cages should be designed in such a way that the confined subject can show natural vertical flight responses (International Primatological Society 1993).
Cage space requirement stipulations in the USA are not congruent with these professional standards because they only partially define the quality of the minimum cage space they prescribe. The rules define the social but not the arboreal quality of the minimum cage space. Taking the social life-style of non-human primates into account, compatible companionship must be provided and the floor area of the cage increased accordingly (United States Department of Agriculture 1991). The arboreal life-style of non-human primates, however, is not taken into account. No provision is made that adequate space and structure be provided to make the vertical dimension of the cage accessible, and suitable for the expression of species-typical arboreal activities and postures and species-typical vertical flight responses. This circumstance contradicts the USA guidelines stipulating that the environment in which an animal is held should be appropriate to the species and its life history (National Institutes of Health 1985). A legal-sized cage will not be appropriate to any non-human primate if it is empty, without means to actively use the vertical space in species characteristic ways. When an elevated structure such as a perch is available, non-human primates will spend up to 90 per cent of their time above the ground exhibiting species-typical arboreal postures and activities (Reinhardt & Smith 1988; Reinhardt 1989, 1990; Schmidt et al 1989; Watson 1991).
Explicitly stipulating that all cages have to be equipped with elevated structures appropriate to each species and age category, would make the US cage space stipulations more adequate. They would be a safeguard against animals being restricted to an unnatural, permanent terrestrial lifestyle. Elevated structures are too important to be merely listed among other options for environmental enrichment (United States Department of Agriculture 1991). Multiple spatial and behavioural benefits derived from elevated structures suggest that they promote the caged primate's state of well-being: they increase the usable cage volume, provide means for species-typical arboreal behaviours (cf. Figure 1) and offer dry places during routine cage-cleaning procedures and 'safe' locations to retreat during fear-inducing situations (O'Neill 1987; Reinhardt & Smith 1988; Reinhardt 1990; Watson 1991; Reinhardt et al 1992).
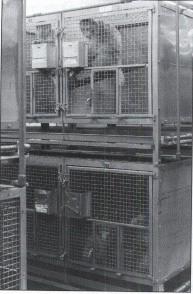
Sitting in an elevated position fosters a monkey's feeling of security by having better visual control over the environment (cf. Figure 1; Estrada 1989; Reinhardt 1989; Woodbeck & Reinhardt 1991). Monkeys in barren cages can often be found clinging to the cage wall (Shimoji et al 1993), probably in an attempt to attain the arboreal dimension. Clearly, the provision of a comfortable perch or shelf is preferable to having to struggle to hold on to the perpendicular wall of the cage. 'Structural enhancement' (Schapiro et al 1991) is particularly important for animals that are confined in dark lower-row cages, and hence forced to continuously live close to the ground. These animals have a definitive disadvantage in comparison with those living in bright upper-row cages (cf. Figure 1) and they consequently make more use of elevated structures, such as perches (Reinhardt 1989; Woodbeck & Reinhardt 1991; Shimoji et al 1993).
In order to guarantee minimum quality standards of vertical cage space for non-human primates, height requirements will have to be upgraded in the US (Figure 1) to allow adequate installation of elevated structures, such as perches, shelves, ledges or swings. Ideally the cage should extend from floor to ceiling of the room thus taking maximum advantage of the space and light available, allowing the installation of several elevated structures at different levels, and making it possible for the occupant(s) to retreat above human eye level in the event of alarm (International Primatological Society 1993).
Animal welfare implications
Legal cage space requirements for non-human primates are not adequate unless they stipulate that sufficient height be provided to accommodate properly placed elevated structures. The provision of such structures promotes the caged subject's well-being by fostering the expression of his/her biological adaptation to a three-dimensional arboreal environment.
Acknowledgements
We are thankful to Klan Fajzi and Annie Reinhardt for critically reading this manuscript and providing constructive comments. The final version of this paper greatly benefited from very valuable suggestions from three anonymous referees of Animal Welfare.
References
American Medical Association 1992 White Paper: Use of Animals in Biomedical Research. The Challenge and Response. Group on Science and Technology American Medical Association: Chicago, USA
Anderson J R and Visalberghi E 1990 Towards better conditions for captive primates: routines, requirements, and research. In: Alleva B and Laviola G (eds) Biomedical Experimentation and Laboratory Animals: Hot Behavioural Issues pp 1-11. Istisan Reports: Rome, Italy
Bayne K A L 1989 Resolving issues of psychological well-being and management of laboratory nonhuman primates. In: Segal EF (ed) Housing, Care and Psychological Well-Being of Captive and Laboratory Primates pp 27-39. Noyes Publications: Park Ridge, USA
Bayne K A L, Hurst J K and Dexter S L 1992a Evaluation of the preference to and behavioral effects of an enriched environment on male rhesus monkeys. Laboratory Animal Science 42: 38-45
Bayne K A L and McCulIy C 1989 The effect of cage size on the behavior of individually housed rhesus monkeys. Lab Animal 18 (1): 25-28
Bayne K, Dexter S, Mainzer H, MeCully C, Campbell G and Yamada F 1992b The use of artificial turf as a foraging substrate for individually housed rhesus monkeys (Macaca mulatta). Animal Welfare 1: 39-53
Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F and Suomi S 1991 The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology 23: 23-35
Benn D M 1995 Innovations in research animal care. Journal of the American Veterinary Medicine Association 205: 465-468 Berkson G 1968 Development of abnormal stereotyped behaviors. Developmental Psychobiology 1:118-132
Berkson G, Mason W A and Saxon S U 1963 Situation and stimulus effect on stereotyped behaviors of chimpanzees. Journal of Comparative Physiological Psychology 56: 786-792
Bernstein I and Mason W 1963 Activity patterns of rhesus monkeys in a social group. Animal Behaviour 12: 338-342
Boinski S, Noon C, Stans S, Samudio R, Sammarco P and Hayes A 1994 The behavioral profile and environmental enrichment of a squirrel monkey colony. Laboratory Primate Newsletter 33 (4): 1-4
Boot R, Leussink A B and VIug R F 1985 Influence of housing conditions on pregnancy outcome in cynomolgus monkeys (Macaca fascicularis). Laboratory Animals 19: 42-47
Bowden D M 1988 Primate research and "psychological well-being". Science 240: 12
Brent L 1992 The effects of cage size and pair housing on the behavior of captive chimpanzees. American Journal of Primatology 27: 20 (Abstract)
Brent L, Lee D R and Eichberg J W 1989 Evaluation of two environmental enrichment devices for singly caged chimpanzees (Pan troglodytes). American Journal of Primatology Supplement 1: 65-70
Brent L and Long K B 1995 The behavioral response of individually caged baboons to feeding enrichment and the standard diet: a preliminary report. Contemporary Topics 34 (2): 65-69
Burt D A and Plant M 1990 Observations on a caging system for housing stump-tailed macaques. Animal Technology 41: 175-179
Canadian Council on Animal Care 1993 Guide to the Care and Use of Experimental Animals, Volume 1. CCAC: Ottawa, Canada
Chamove A S 1989 Environmental enrichment: a review. Animal Technology 40: 155-178
Chamove A S and Anderson JR 1989 Examining environmental enrichment. In: Segal B F (ed) Housing, Care and Psychological Well-Being of Captive and Laboratory Animals pp 183-202. Noyes Publications: Park Ridge, USA
Chopra P K, Seth P K and Seth P K 1992 Behavioural profile of free-ranging rhesus monkeys. Primate Report 32: 75-105
Crockett CM 1993 Primate well-being is not promoted by suit. Laboratory Primate Newsletter 32 (2): 1-2
Crockett C M and Bowden D M 1994 Challenging conventional wisdom for housing monkeys. Lab Animal 24 (2): 29-33
Crockett C M, Bowers C L, Bowden D M and Sackett G P 1994 Sex differences in compatibility of pair-housed adult longtailed macaques. American Journal of Primatology 32: 73-94
Crockett C M, Bowers C L, Sackett G P and Bowden D M 1993a Urinary cortisol responses of longtailed macaques to five cage sizes, tethering, sedation, and room change. American Journal of Primatology 30: 55-74
Crockett C M, Bowers C L, Shimoji M, Leu M, Bellanca R and Bowden D M 1993b Appetite and urinary cortisol responses to different cage sizes in female pigtailed macaques. American Journal of Primatology 31: 305 (Abstract)
Crockett C M and Wilson W L 1980 The ecological separation of Macaca nemestrina and M. fascicularis in Sumatra. In: Lindburg D G (ed) The Macaques: Studies in Ecology, Behavior and Evolution pp 148-181. Van Nostrand Reinhold: New York, USA
Daschbach NJ, Schein M Wand Haines D E 1983 Cage-size effect on locomotor, grooming and agonistic behaviors of the slow loris, Nycticebus coucang. Applied Animal Ethology 9: 317-330
Der Schweizerische Bundesrat 1981 Tierschutzverordnung. Bundesrat: Bern, Switzerland
DeVore I and Hall K R L 1965 Baboon ecology. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 20-52. Holt, Rinehart and Winston: New York, USA
Draper WA and Bernstein I S 1963 Stereotyped behavior and cage size. Perceptual and Motor Skills 16: 231-234
Eaton G G, Kelley S T, Iliff-Sizemore S A 1993 Rawhide "chew-bones reduce abnormal behavior in individually housed adult rhesus macaques. American Journal of Primatology 30: 308 (Abstract)
Erwin J 1991 Applied primate ecology: evaluation of environmental changes to promote psychological well-being. In: Novak M A and Petto A J (eds) Through the Loaking Glass. Issues of Psychological Well-Being in Captive Nonhuman Primates pp 180-188. American Psychological Association: Washington, USA
Erwin J and Deni R 1979 Strangers in a strange land: abnormal behavior or abnormal environments? In: Erwin J, Maple T and Mitchell G (eds) Captivity and Behavior pp 1-28. Van Nostrand Reinhold: New York, USA
Estrada A 1989 Comportamiento Animales. Es Caso de los Primates. La Ciencia desde Mexico: Mexico
European Economic Community Council 1986 Directive 86/609 on the Approximation of Laws, Regulations, and Administrative Provisions Regarding the Protection ofAnimals Used for Experimental and Other Scientific Purposes: Annex II Guidelines for Accommodation and Care of Animals. Official Journal of the European Communities L358: 7-28
Field K J, Denny J and Kubicz G 1992 Nonhuman primate socialization and environmental enrichment using a transfer tunnel. Laboratory Primate Newsletter 31 (2): 5-7
Fittinghoff N A, Lindburg D G, Gomber J and Mitchell G 1974 Consistency and variability in the behavior of mature, isolation-reared, male rhesus macaques. Primates 15: 111-139
Fritz J, Nash L T, Alford P L and Bowen J A 1992 Abnormal behaviors, with special focus on rocking, and reproductive competence in a large sample of captive chimpanzees (Pan troglodytes). American Journal of Primatology 27: 161-176
Goodall J 1965 Chimpanzees of the Gombe Stream Reserve. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 425-473. Holt, Rinehart and Winston: New York, USA
Goosen C 1988 Developing housing facilities for rhesus monkeys: prevention of abnormal behavior. In: Beynen A C and Solleveld H A (eds) New Development in Biosciences: Their Implications for Laboratory Animal Science pp 67-70. Martinus Nijhoff: Dordrecht, The Netherlands
Hediger H 1955 Studies of the Psychology and Behaviour of Animals in Zoos and Circuses. Butterworths: London, UK
Holden C 1988 Experts ponder simian well-being. Science 241:1753-1755
Home Office 1989 Animals (Scientific Procedures) Act 1986. Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. HMSO: London, UK
International Primatological Society 1993 IPS international guidelines for the acquisition, care and breeding of nonhuman primates. Primate Report 35: 3-29
Jay P 1965 The common langur of North India. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 197-249. Halt, Rinehart and Winston: New York, USA
Kaplan M L and Lobao B J 1991 A unique housing system for rhesus macaques. Lab Animal 20 (6): 48-51
Ken J and Rothe H 1996 Influence of cage size and cage equipment on physiology and behaviour of common marmosets (Calhthnx jacchus). Laboratory Primate Newsletter 35(3): 10-13
Lam K, Rupniak N M J and Iversen S D 1991 Use of a grooming and foraging substrate to reduce cage stercotypies in macaques. Journal of Medical Primatology 20: 104-109
Leu M, Crockett C M, Bowers C L and Bowden D M 1993 Changes in activity levels of singly housed longtailed macaques when given the opportunity to exercise in a larger cage. American Journal of Primatology 31: 327 (Abstract)
Line S W 1987 Environmental enrichment for laboratory primates. Journal of the American Veterinary Medical Association 190: 854-859
Line S W, Markowitz H, Morgan K N and Strong S 1989a Evaluation of attempts to enrich the environment of single-caged non-human primates. In: Driscoll J W (ed) Animal Care and Use in Behavioral Research: Regulations, Issues, and Applications pp 103-117. Animal Welfare Information Center: Beltsville, USA
Line S W, Markowitz H, Morgan K N and Strong S 1991 Effect of cage size and environmental enrichment on behavioral and physiological responses of rhesus macaques to the stress of daily events. In: Novak M A and Petto A J (eds) Through the Looking Glass. Issues of Psychological Well-Being in Captive Nonhuman Primates pp 160-179. American Psychological Association: Washington, USA
Line S W, Morgan K N, Markowitz H, Roberts J and Riddell M 1990b Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Laboratory Primate Newsletter 29(4): 1-5
Line S W, Morgan K N, Markowitz H and Strong S 1989b Influence of cage size on heart rate and behavior in rhesus monkeys. American Journal of Veterinary Research 40: 1523-1526
Line S W, Morgan K N, Markowitz H and Strong S 1990a Increased cage size does not alter heart rate or behavior in female rhesus monkeys. American Journal of Primatology 20: 107-113
Martin R D 1990 Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton University Press: Princeton, USA
Mason WA 1989 Primatology and primate well-being. American Journal of Primatology Supplement 1: 1-4
Menzel E W 1967 Naturalistic and experimental research on primates. Human Development 10: 170-186
Meunier L D, Duktig J T and Landi M S 1989 Modification of stereotypic behavior in rhesus monkeys using videotapes, puzzlefeeders, and foraging boxes. Laboratory Animal Science 39: 479 (Abstract)
Nadler R D, Herndon J G, Metz B, Ferrer A C and Erwin J 1992 Environmental enrichment by varied feeding strategies for individually caged young chimpanzees. In: Chimpanzee Conservation and Public Health: Environments for the Future pp 137-145. DiagoniBioqual: Rockville, USA
National Institutes of Health 1985 Guide for the Care and Use of Laboratory Animals. NIH Publication No 85-23: Bethesda, USA National Institutes of Health 1991 Nonhuman Primate Management Plan. NIH: Bethesda, USA
O'Neill P 1987 Enriching the lives of primates in captivity. Humane Innovations and Alternatives in Animal Experimentation 1: 1-5
O'Neill-Wagner P 1994 When trying to get your monkeys to behave, try perches. In Touch 1 (2): 6-8
Perkins S E, Burnett D E, Rice T R, Staley E C and Weick B G 1992 Evaluation of the use of novel objects by adult male Macaca mulatta, singly housed in Horafal isolators. Laboratory Primate Newsletter 31 (4): 5-7
Phillippi-Falkenstein K 1993 Responses of singly-housed white-crowned mangabeys (Cercocebus torquatus lunulatus) to different enrichment devices. Laboratory Primate Newsletter 32 (4): 5-7
Poole T B 1992 The nature and evolution of behavioural needs in mammals. Animal Welfare 1: 203-220
Reinbardt V 1989 Evaluation of the long-term effectiveness of two environmental enrichment objects for singly caged rhesus macaques. Lab Animal 18(6): 31-33
Reinhardt V 1990 A perch for caged macaques. Humane Innovations and Alternatives in Animal Experimentation 4: 134-135
Reinhardt V 1991 Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. Journal of Experimental Animal Science 34: 73-76
Reinhardt V 1992 Space utilization by captive rhesus macaques. Animal Technology 43: 11-17
Reinhardt V 1995 Environmental Enhancement for Caged Macaques: A Photographic Documentation. Animal Welfare Institute: Washington, and Wisconsin Regional Primate Research Center: Madison, USA
Reinhardt V, Houser W D, Cowley D and Champoux M 1987a Preliminary comments on environmental enrichment with branches for individually caged rhesus monkeys. Laboratory Primate Newsletter 26 (1): 1-3
Reinhardt V, Houser W D, Eisele S G and Champoux M 1987b Social enrichment of the environment with infants for singly caged adult rhesus monkeys. Zoo Biology 5: 365-371
Reinhardt V and Smith MD 1988 PVC pipes effectively enrich the environment of caged rhesus monkeys. Laboratory Primate Newsletter 27 (3): 4-5
Reinhardt V, Zweifel D and Pape D 1992 Improving the micro environment of caged laboratory macaques. Animal Technology 43: 179-183
Reynolds V and Reynolds F 1965 Chimpanzees of the Budongo Forest. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 368-424. Holt, Rinehart and Winston: New York, USA
Rose M D 1974 Postural adaptations in New and Old World monkeys. In: Jenkins F A (ed) Primate Locomotion pp 201-222. Academic Press: New York, USA
Rosenbium L, Kaufman I and Stynes A 1964 Individual distances in two species of macaques. Animal Behaviour 12: 338-342
Ruempler U 1992 Besch1ftigungsmo~glichkeiten bei Primaten im Zoo. Zeitschrift des Kölner Zoo 35 (2): 47-68
Salzen E A 1989 A closed colony of squirrel monkeys for laboratory studies. In: Segal E F (ed) Housing, Care and Psychological Well-Being of Captive and Laboratory Primates pp 115-134. Noyes Publications: Park Ridge, USA
Schapiro S J, Bloomsmith M A, Kessel A L and Shively C A 1993 Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science 37: 251-263
Schapiro S J, Brent L, Bloomsmith M A and Satterfield W C 1991 Enrichment devices for nonhuman primates. Lab Animal 20 (6): 22-28
Schmidt E M, Dold G M and McIntosh J S 1989 A perch for primate squeeze cages. Laboratory Animal Science 39:166-167
Schultz A H 1969 The Life of Primates. Universe Books: New York, USA
Shimoji M, Bowers C L and Crockett C M 1993 Initial response to introduction of a PVC perch by singly caged Macaca fascicularis. Laboratory Primate Newsletter 32 (4): 8-11
Simonds P E 1965 The bonnet macaque of South India. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 175-196. Holt, Rinehart and Winston: New York, USA
Taff M A and Dolhinow P 1989 Langur monkeys (Presbytis entellus) in captivity. In: Segal EF (ed) Housing, Care and Psychological Well-Being of Captive and Laboratory Primates pp 291-304. Noyes Publications: Park Ridge, USA
Traylor-Holzer K and Fritz P 1985 Utilization of space by adult and juvenile groups of captive chimpanzees (Pan troglodytes). Zoo Biology 4:115-127
United States Department ofAgriculture 1991 Animal welfare; standards; final rule. Federal Register 56: 6426-6505 Vandenbergh J G 1989 Issues related to "psychological well-being" in nonhuman primates. American Journal of Primatology 1: 9-15
Washburn R and Hamburg D A 1965 The implications of primate research. In: DeVore I (ed) Primate Behavior: Field Studies of Monkeys and Apes pp 607-629. Holt, Rinehart and Winston: New York
Watson D SB 1991 A built-in perch for primate squeeze cages. Laboratory Primate Newsletter 41: 378-379
Watson S L and Shively C A 1996 Effects of cage configuration on behaviour in cynomolgus macaques. International Primatological Society/American Society of Primatologists Congress. Abstract No 674
Weed J L, Baker S C, Harbaugh S W and Erwin J 1995 Innovative enclosures for laboratory primates: evaluation of a "breeding condominium". Lab Animal 24 (7): 28-32
Weld K and Erwin J 1990 Provision of manipulable objects to cynomolgus macaques promotes species-typical behavior. American Journal of Primatology 20: 243 (Abstract)
Weld K, Metz B and Erwin J 1989 Experimental evaluation of environmental enrichment techniques: provision of manipulable objects to five primate species. American Journal of Primatology 18: 169 (Abstract)
Wemelsfelder F 1994 Animal boredom - a model of chronic suffering in captive animals and its consequences for environmental enrichment. Humane Innovations and Alternatives 8: 587-591
Whitney R A and Wickings E J 1987 Macaques and other old world simians. In: Poole TB (ed) The UFA W Handbook on the Care and Management of Laboratory Animals pp 599-627. Churchill Livingstone: New York, USA
Williams L E and Abee C R 1985 Cage design and configuration for an arboreal species of primate (genus Saimiri). Laboratory Animal Science 35: 529 (Abstract)
Wilson S F 1982 Environmental influences on the activity of captive apes. Zoo Biology 1: 201-209
Wolfle T L 1991 Psychological well-being: the billion-dollar situation. In: Novak M A and Petto A J (eds) Through the Looking Glass. Issues of Psychological Well-Being in Captive Nonhuman Primates pp 119-128. American Psychological Association: Washington, USA
Woodbeck T and Reinhardt V 1991 Perch use by Macaca mulatta in relation to cage location. Laboratory Primate Newsletter 30 (4): 11-12
Woolverton W L, Ator N A, Beardsley P M and Carroll M E 1989 Effects of environmental condition on the psychological well-being of primates. A review of the literature. Life Sciences 44: 901-917
Published in Animal Welfare 5(4), 361-372 (1996)
© 1996 Universities Federation for Animal Welfare