Viktor Reinhardt
Animal Welfare Institute, Washington, DC
4605 Crescent Road, Madison, WI 53711
Many primatological scientists, investigators, veterinarians and colony managers are over-afraid of nonhuman primates, and therefore condone that the animals be forcefully restrained during procedures requiring any form of physical human-animal interaction.
So-called squeeze-backs are used to push an animal to the front of its cage and immobilize it during blood collection, injection or other procedures. Squeeze-backs are also used to make an animal leave its cage and enter a transfer box. Nets are used to catch individual animals of groups living in pens or corrals. Restraint chutes with adjustable restrictors and/or guillotine panels are used to immobilize an animal during sample collection, injection, treatment, and other procedures (Figure 1). Tables or specially designed boards or crosses are used to manually or mechanically hold an animal in the so-called crucifixion position for sample collection, electrocardiographic recording and other procedures. Pillory type attachments at the neck and waist are used to keep an animal in a seated positioned during manipulations of any parts of its body, sample collection, psychological testing, and other procedures.
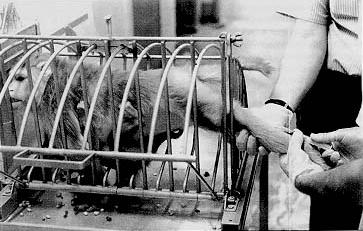
Nonhuman primates often struggle when being restrained, not only because of the physical discomfort but because of adverse conditioning with restraint being the predictable prelude to an uncontrollable, usually painful procedure. Being removed from the familiar homecage during most restraint situations adds to the anxiety experienced and subjects often show gross signs of distress such as acute diarrhea, rectal prolapse, and alarm vocalization.
Some investigators have long recognized that involuntary restraint introduces stress-related physiological variables confounding scientific research data. Typical physiological responses to restraint include increased cortisol and catecholamine secretion, elevated heart rate and blood pressure, pronounced leukocytosis, and immune suppression. Some responses persist even if several weeks are allowed for habituation. This suggests that primates held in laboratories do no adjust to being forcefully restrained and supports the regulatory recommendations of limiting restraint to no longer than 12 hours (USDA, 1991) or avoid restraint altogether (CCAC, 1993). Unfortunately, these recommendations are not binding, and keeping nonhuman primates under continuous involuntary restraint conditions for much longer than " only" 12 hours is not an uncommon practice.
Compulsory restraint not only prompts scientific reservations related to the validity of research data collected from the distressed subject, but it also raises ethical concerns. It is one of the U.S. Government Principles (1985) that aptly calls upon the investigator to "consider that procedures that cause pain or distress in human beings may cause pain or distress in other animals." The observable and measurable distress responses give scientific evidence that the forcefully restrained nonhuman primate suffers similar distress as the human primate would do under the same circumstance. Are there any alternatives?
The British Code of Practice for the Housing and Care of Animals Used in Scientific Procedures (1989) points out that the least distressing method of handling is to train the nonhuman primate to cooperate during the procedure.
I became aware of the feasibility of training laboratory primates in 1988 while observing a senior animal caregiver, Russell Vertein, of the Wisconsin Regional Primate Research Center, working with a female rhesus macaque. He gently talked to the animal and offered her raisins through the mesh of the cage while trying to get hold of her leg through the partly opened cage door. Russell did not like the traditional venipuncture technique, which would require removing the animal from the cage and manually restraining it on a table with the help of another person or mechanically in a chute. He felt bad forcefully subduing these sensitive animals day after day and seeing them fearfully resisting the handling. Taking the animals' intelligence and social nature into account, Russell had no doubt that it should be possible to train them so they could be handled in their familiar homecages without being distressed.
The idea appealed to me, and we developed a positive-reinforcement training protocol and tested it in eight adult, pair-housed female rhesus macaques. On average, 31 minutes of cumulative training time was required until a female showed no signs of fear or resistance to having a leg gently pulled through the cage door and the saphenous vein punctured (Vertein & Reinhardt 1989). It was encouraging to see how easily the animals learned to cooperate and quickly overcame their fear. I therefore decided to further refine the training technique and test it in adult male rhesus macaques who have the reputation of being particularly intractable. Five single-housed and 10 pair-housed males were the subjects of the study. On average, 13 training sessions were necessary to get a male to voluntarily present a leg in a specially designed opening of the door and to display no resistance during venipuncture (Figure 2). Serum cortisol analysis confirmed the subjective impression that the animals were not distressed (Reinhardt et al., 1991). Total time spent with a male until he actively cooperated ranged from 16 to 74 minutes, with a mean of 40 minutes (Reinhardt, 1991). This time investment paid off quickly in the avoidance of unnecessary distress, more reliable research data and a more satisfying work environment. Working with, rather than against an animal also increased my own safety and that of the subject because the interaction was based on trust rather than fear. I tested the training technique in 31 female rhesus macaques and in eight female stumptailed macaques with the same result: Less than one hour total training time per female was required to assure that individuals actively cooperated during in-homecage venipuncture without showing measurable signs of distress.

Blood collection is the handling procedure nonhuman primates are most often subject to. Strictly implementing in-homecage venipuncture training programs would markedly refine scientific methodology while eliminating a major cause of distress inflicted on primates assigned to research.
Primatological investigators often underestimate their research subjects' level of intelligence when they hesitate to train rather than restrain them. They should capitalize on the animal's intelligence for the sake of more reliable research methodology. A search of the scientific literature shows that nonhuman primates can readily be conditioned not only to cooperate during venipuncture but also during capture from a group, removal from the homecage, systemic, topical and oral in-homecage drug application, in-homecage urine collection, and in-homecage veterinary examination (Figure 3). With some professional expertise and goodwill, there should be no real need to resort to forceful restraint when doing research with nonhuman primates. The animals deserve a more humane treatment.

References
British Codes of Practice for the Housing and Care of Animals Used in Scientific Procedures (1989). Home Office Animals (Scientific Procedures) Act 1986. London.
Canadian Council on Animal Care (1993). Guide to the Care and Use of Experimental Animals, Vol. 1. Ottawa, Ontario: CCAC.
Reinhardt, V.; Cowley, D. (1990). "Training stumptailed monkeys to cooperate during in-homecage treatment." Laboratory Primate Newsletter, 29(4), 9-10.
Reinhardt, V. (1991). "Training adult male rhesus monkeys to actively cooperate during in-homecage venipuncture." Animal Technology, 42, 11-17.
Reinhardt, V.; Cowley, D.; Eisele, S.; Scheffler, J. (1991). "Avoiding undue cortisol responses to venipuncture in adult male rhesus macaques." Animal Technology, 42, 83-86.
U.S. Government Principles (1985). "Principles for the utilization and care of vertebrate animals used in testing, research, and training." Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23), 82-83. U.S. Department of Health and Human Services, Washington, DC.
U.S. Department of Agriculture (1991). "Animal Welfare, Standard, Final Rule." Federal Register, 56, 6499-6500.
Vertein R.; Reinhardt, V. (1989). "Training female rhesus monkeys to cooperate during in-homecage venipuncture. Laboratory Primate Newsletter, 28(2), 1-3.
Fall 1994 IN TOUCH
This article has been reproduced with the permission of the publisher.