Schnell, C.R. And Gerber, R.
Key words: Primate, Callithrix jacchus, Macaca mulatta, Radiotelemetry, Chronic monitoring, blood pressure, heart rate, stress
Summary
It is now well accepted that acclimation to handling (or gentling) in non-humans primates can have a significant impact on the quality of data measured by reducing variance and increasing the significance level of observed changes. However, relatively few well controlled studies on this topic have been published. The use of "pre-invasive" implantable radio telemetry has revolutionized the collection of physiological data under stress-free conditions. It is now possible to measure accurately 'normal' baseline data of haemodynamic and electrical parameters in conscious and unrestrained monkeys. The use of this new technology enabled us to measure the pronounced acute and chronic effects on cardiovascular parameters of singly or repeated oral administration procedures in trained marmosets. Advantages, as well as limitations, of alternative "low-stress" ways of dosing will be discussed. It is frequently suggested that training is not "cost-effective". Data from our own studies have demonstrated unequivocally the impact of housing/husbandry practices on physiological parameters and pharmacological outcome.
Introduction
Much has been done over the past 20 years to improve animal care and well-being and also to improve the quality of research data. However, most areas of animal research require interaction between the animal and the research staff and this often involves handling. Such interactions can cause marked changes to commonly measured biological parameters such as serum hormones, tissue metabolites levels, heart rate and blood pressure (Gartner, 1980). Training (or gentling) is of special importance in experimental studies involving non-human primates. It has been reported in the literature that non-human primates unaccustomed to an experimental situation, exhibit external behavioral manifestations of excitation and develop considerably less obvious changes in a number of physiological functions (Lapin, 1963).
However, there are relatively few published data on the effects of handling and gentling in non-humans primates. Only 3 % of all the papers published in the last 15 years on the use of non-human primates in biomedical research have addressed this specific topic. This can be related to the difficulties encountered by animal researchers in obtaining meaningful basal control values (which are pivotal for such studies) without disturbing the animal involved (for example by restraint, catheterisation, isolation or presence of the experimenter).
Recent advances in "pre-invasive" implantable radio telemetry has revolutionized the collection of physiological data under stress-free conditions. Haemodynamic and electrical parameters can now be accurately and reliably measured in conscious freely moving monkeys.
In this report we presents 'normal' baseline data for mean arterial pressure (MAP) and heart rate (HR) obtained via radio-telemetry in rhesus macaques (Macaca mulatta) and marmosets (Callithrix jacchus) maintained in their home cages.
Moreover, recent results obtained in our laboratory will be used to demonstrate the pronounced acute and chronic effects on cardiovascular parameters of singly or repeated oral administration procedures in marmosets. Advantages, as well as limitations, of alternative "low-stress" ways of dosing will be presented.
Methods
Telemetry system
The telemetry system (Data Sciences Int., St Paul, Minnesota) used in this studies for the measurements of cardiovascular parameters consists of 4 basic components as described previously (Schnell & Wood, 1993): an implantable transmitter (AM unit, model TA11PA-C40 or PA-D70 for the marmosets and rhesus macaques respectively), a receiver located within the cage, a matrix interface for coordination of signals and a computer-based data acquisition system for collection analysis and storage of data. The pressure transmitters were implanted into the peritoneal cavity of the marmosets or subcutaneously on the flank of the rhesus macaques. The sensor catheter was placed in the aorta of the marmosets or in a branch of the femoral artery of the rhesus monkeys.
The analyses of the signal was made using a Dataquest analysis routine (PhysioStat Analysis, Data Sciences). BP is expressed in millimeters of mercury (mmHg), HR in beats per min (bpm) and motor activity in number of movements (units) per 2 min.
Statistics
The average values of data collected over the time period of observation were calculated for individual animals. For comparisons within groups, statistical analyses were performed on averaged data using the two-tailed Student's t-test for paired data or the unpaired test for unpaired data. Values given in text and figures are means B1 SE. The probability 0.05 was taken as statistically significant.
Results
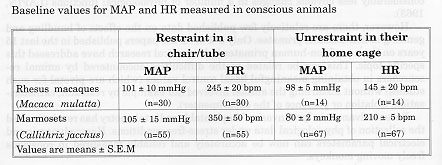
All the measurements obtained in restraint monkeys were performed in animals previously trained to become accustomed to the measurement procedure. Both parameters were lower under unrestrained conditions when compared to the restraint situation. HR was nearly 100 beats per minutes (bpm) lower in the unrestrained situation for both species. Because increase in HR is one of the major cardiovascular response to stress as previously described in the literature (Herd, 1991), we could demonstrate very clearly how stressful restraint can be to non-human primates, even when they were trained to the experimental procedure over a long time before. Similar observations have been done in cynomolgus monkeys (Mann, 1991).
The acute effect of stress due to restraint may have large implications for the evaluation of the pharmacological profile of a drug in biomedical research.
Can acute stress influence drug activity?
We conducted a study in order to compare the BP lowering action of an Angiotensin-II (AII) antagonist measured either in tube restraint or in freely moving marmosets maintained in their home cages. MAP and HR were recorded continuously using radio-telemetry for a 6 hours period after acute dosing of the All antagonist by oral gavage. Application of different doses were randomized for both groups and given at different days. Drug efficacy was calculated using the area under the curves (AUC; delta mmHg x 6 hours) determined by the trapezoidal rule method.
A clear shift to the right was observed in the dose response curve obtained under unrestrained conditions when compared to the restraint situation (Fig. 1). The potency of the All antagonist based on the BP lowering activity was found to be more then 10 times higher when tested in restraint marmosets. Moreover, the pronounced reflex tachycardia (+ 90 bpm) we could observed during the first 2 hours after the 10 mg/kg p.o. dose of the AII-antagonist in unrestrained marmosets was not observed under restrained conditions. This is probably because the initial values for HR recorded in restrained marmosets where much higher (390 ± 18 bpm, n=7; 205 ± 13 bpm, n=9, respectively, for restraint and unrestrained marmosets) compare to the freely moving situation
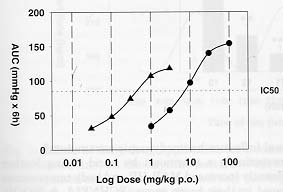
Fig. 1: Dose response curve of an Angiotensin-II antagonist measured either in tube restraint (triangles) or in freely moving marmosets (squares) maintained in their home cages. BP lowering activity of the drug based on the IC50 was found to be more then 10 times higher when tested in restraint marmosets (IC50: 0.3 mg/kg p.o., n=6 to 8 per group; 8 mg/kg p.o., n=6 to 8 per group, respectively, for restraint and unrestrained marmosets).
Can chronic stress influence haemodynamic parameters?
In our facilities, marmosets are acclimated to be routinely involved in experimental procedures in a monthly turnout. They have a resting period of 3 weeks between the experiments. During the experiments MAP and HR are recorded telemetrically in animals freely moving in their home cage. Under this conditions, 'true' physiological baseline values can be obtained. Both parameters showed a remarkable stability for a period up to 2 to 3 years. For a period of approximately 9 months, the marmosets were not involved in any experimental procedure. Thereafter, the experiments started again as described previously. It was very interesting for us to see that the first baseline values of HR recorded after this 9 month break was significantly lower compare to the last pre-break recording (213 ± 7 bpm vs 180 ± 7 bpm, n=42, respectively, before and after the break, Fig. 2). However, HR returned to pre-break initial values when the third following experiment was performed suggesting the presence of a constant background stress level in our marmosets when they are routinely involved in drug application procedures. MAP showed no significant changes between pre and post-break recordings.
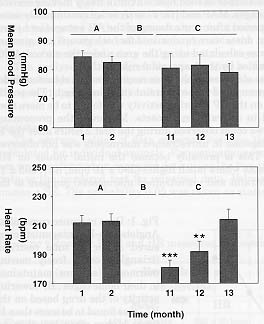
Fig. 2: Effects of routine drug application procedures on mean blood pressure (n=14) and heart rate (n=14) in trained conscious marmosets freely moving in their home cages. Hatched bars, initial values measured between 9 A.M. and 10 A.M. before dosing. A, pre-break values. B, 9 months break. C, post-break values. Values represent means ± SEM. P<0.005 = ***, P<0.01 = **, P<0.05 = *.
How can dosing a pre-trained animal influence haemodynamic parameters?
We reported previously that restraining a marmoset by hand (using leather gloves) for a period of 1 min. significantly increased MAP, HR and body temperature even after the animals were replaced in their home cages (Schnell & Wood,1993). All the parameters normalized after a 30 minutes post-restraining period. In the present study we wanted to analyze if marmosets can adapted to the experimental procedure of oral gavage. For this purpose, 12 marmosets were dosed daily (at 10 a.m.) with honey water for 4 consecutive weeks. They were directly catch in their home cage using a butterfly net. Dosing was performed using a children feeding tube. It takes between I and 2 minutes for an experimented team to go through this procedure. We observed a significant increase in hourly mean values for systolic (SBP), diastolic (DBP) blood pressure and HR in the first hour after dosing (Fig.3).
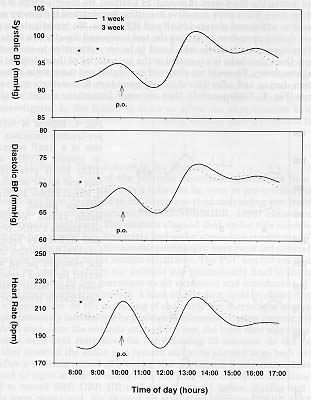
Fig. 3: Effects of daily drug application for 3 consecutive weeks (1 week: solid line, 3 week: dotted line) on the daytime pattern of systolic BP, diastolic BP and HR (n=12) in pre-trained conscious marmosets freely moving in their home cages. P < 0.005 = ***, P < 0.01 = **, P < 0.05 = *.
Values normalized during the second post-dosing hour. During the third week of daily application, initial baseline values before dosing were already stimulated to first week maximum levels. No additional post- dosing increase effect was observed for all the measured parameters. Similar observations were performed during the fours week of application. These results showed that marmosets could not adapted to the stress of the experimental procedure. Moreover, they seemed to anticipate the dosing event with time, which could lead to the development of a sustained cardiovascular stress pattern as suggested by the increased initial baseline HR values when measured during the third week of dosing. In addition, we observed that marmosets at this stage were much more sensitive to routine housing and husbandry activities which leads to additional elevated MAP and HR values. We investigated a possible 'low stress' dosing procedure by mixing the test compound directly in a banana-milk shake. This banana-milk shake was found to be very attractive to our marmosets over years. Once the shake is presented to the animals, all of them drink it immediately and completely. We could not observed any significant increase in all measured parameters during and after this drinking procedure demonstrating a very good acceptance (Fig. 4). Unfortunately, they are some limitations of this 'ideal' way of dosing. The animals have to be isolated during the dosing procedure to ensure that the right animal took the right amount of drug. However, the major limitation we encountered in performing experiments using this voluntary procedure of dosing was that if the drug tested presented unpleasant effects to the marmosets (like headache, vertigo,...) they refused with time to drink there 'favorite' milk shake. For example we conducted a study dosing the animals once a day with an inhibitor of nitric oxide synthase which increased the MAP by 23 mmHg at peak for 6 hours. HR was decreased by 60 bpm at peak in the same time period. Under this conditions, more then 50 % of all marmosets involved in this study stopped drinking the shake after 4 weeks of treatment. They was a clear correlation between the intensity of drug effect observed in an animal and his decision to stop drinking the milk-shake. We have collected some preliminary data using a soft-restraining method to dose our animals. It consists of gently catching the marmoset by hand (using cotton gloves), maintaining the animal in one hand and presenting the milk-shake in a syringe with the other hand. Under this conditions the animals showed no obvious signs of fear and we could not detected any elevation of MAP and HR after restraint and dosing. However, additional alternative 'low stress' way of dosing non-human primates need to be investigated in the future in order to allow the experimenter to conduct a pharmacological study using remote monitoring without having to handle the animal at all.
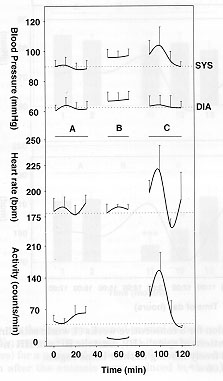
Fig. 4: Effects of restraining pre-trained conscious marmosets in a small compartment on systolic (SYS) , diastolic (DIA) blood pressure, heart rate and motor activity (n=6). A, initial values. B, 20 minutes restrain period. C, post-restrain values. Values represent means ± SEM. No significant differences between values obtained before or during the restraint period were observed for blood pressure and heart rate. Motor activity was significantly lower (p<0.005).
Does restraint always cause distress to the animals?
We have shown in most of the previous described experiments how stressful restraining an animal by hand or in a tube can be, even in pre-trained marmosets. However, it has been described in the literature that restraining pre-trained single marmosets in a small compartment (Anzenberger, 1993) caused no obvious signs of stress or panicking. The authors claimed that under this conditions urine samples can be collected non-invasively and under stress-free conditions. We decided to conduct a study where cardiovascular parameters were recorded telemetrically in 6 marmosets restrained under similar conditions. The PVC box (28 cm W x 40 cm H x 20 cm D) we used to restrain our animals was continuously fixed to the home cage and frequently used by the marmoset as an extension and enrichment of the cage. One side of the box was transparent. Aback sliding door could be closed from outside by the experimenter. At the beginning of the study the marmosets were trained to enter spontaneously into the box by positive reinforcement using banana milk-shake. In a second step, once the animals entered the box, the back door was closed and the marmosets were kept restrained for the following 20 minutes. At the end of this period they received the shake as a reward and were allowed to go back to the home cage. After a training period of 3 weeks, the marmosets freely entered the box and presented no signs of fear or stress once the door was closed. At this time point we started to record SBP, DBP, HR and motor activity before, during and after the animals were restrained. We showed no statistical increase in SBP, DBP and HR during the whole restraining period. There was a clear and expected fall in motor activity during the entire restraining phase of the experiment. Once the animals were allowed to return to their home cages, there was a significant increase in SBP and HR for the first 10 minutes. This was related to the tremendous increase in motor activity we observed at this time point, mainly due to social interactions activities with the partner after re-pairing. Based on this cardiovascular results, we can postulate that maintaining a pre-trained marmoset in a small space for a limited time period is not necessarily a stressful event. Additional endocrinological studies needs to be done to confirm this assumption.
Discussion
The measurements of control values of biological parameters in experimental non-human primates is always complicated by the degree of interference required to take the measurements. Since the purpose of physiological recording should be to obtain a record that is an exact facsimile or analog of the events under investigation, stress induced by restraint and handling, even when these are of minor nature and performed by skilled staff, is one of the major problem encountered in biomedical investigations. It may be that many animal stress studies involving restraining and handling non-human primates have observed only a small increase in the levels of corticosteroids because the animals were already under stress and therefore had elevated initial hormone levels in the blood. Nonetheless, it has been proposed that training (or gentling) of non-human primates to experimental situation increases cooperative behaviour, decreases behavioral components of defense, enhances staff safety and minimizes animal's distress. However, assessments of moderate stress in animal research is not easy and most of the time relies on subjective research scientists judgments.Therefore, high values of some physiological functions are often recorded when monkeys appear to be calm. It can be anticipated that absence of restriction and especially of man's direct presence would lead to full calm and normalization of physiological parameters. Recent advances in telemetry makes it possible to verify this assumption. Presently, commercially available radio-transmitters allow remote monitoring of haemodynamic and electrical parameters in a wide range of non-human primates maintained in their home cage and without the presence of humans. The present data demonstrate that under this conditions, 'true' physiological basal values for MAP and HR could be obtained in rhesus macaques and in marmosets. However, our experience has shown that, as far as HR is concerned, it is impossible to completely inhibit the defense and orientating reaction to experimental procedures in marmosets even in case of a long-term training. Thereafter, initial normal values for physiological parameters should always be related to the corresponding housing/husbandry practices. We were able to evaluate the impact of restraining procedures on the pharmacological profile of an AII-antagonist tested in marmosets. Moreover, we could demonstrate that even when the marmosets were acclimated to the experimental situation and restriction (during years), dosing still caused marked acute and chronic changes in MAP and HR. Assuming that the intensity of the stress response depends on the intensity of mental effort exerted to meet a challenging situation, whether or not that situation is perceived as threatening, we investigated several 'low-stress' dosing approaches. We could show that dosing a marmoset in his home cage without handling, caused no elevations in MAP and HR. However, under this conditions the research scientist completely relies on the willingness of the animals to take or not to take the presented drug at a given time point. This is incompatible with experiments that have to be performed under G.L.R (Good Laboratory Practice) conditions. One alternative dosing procedure is currently under investigation in our primate facilities. It involves the combination of 'soft handling' and spontaneous drinking behaviour. It is more time consuming for the staff but preliminary data have already shown encouraging results in reducing distress to the animals. Finally, we could prove that marmosets can be trained to participate willingly in cage restraint and to urine sampling by the use of appropriate (banana milk-shake) rewards. Under this prospects, they showed no behavioral and cardiovascular signs of distress.
In summary, our results show the advantage of remote monitoring for making accurate and reliable measurements of haemodynamic parameters in conscious non-human primates freely moving in their home cage. The combination of such refinement of measurement techniques and training of non-human primates to handling and experimental procedure will reduce the variance and increase the significant level of observed changes, allows the measurement of normal physiological parameters and finally reduces the number of animals used in an experiment. Any approach that reduces research animals distress should answer both the need for valid data and the call for improved care and welfare of the animals. Remote and "pre-invasive" monitoring provide opportunities for such assessments and further studies are urgently needed to enable 'best-practice' to be identified using objective criteria.
Acknowledgments
We thank Dr. Yvon Fradin, Roussel-Uclaf, Romainville, France for providing the data obtained via radio-telemetry in rhesus macaques (Macaca mulatta).
References
Anzenberger, G. and Gossweiler, H.: How to obtain individual urine samples from undisturbed marmoset families. American Journal of Primatology (1993) 31: 223-230.
Gartner, K., Buttner, D., Dohler, K., Friedel, R., Lindena, J. and Trautschold, I.: Stress response of rats to handling and experimental procedures. Lab. Anim. (1980) 14: 267-274.
Herd, J.A.: Cardiovascular response to stress. Physiological Reviews (1991) 71 M: 305-330.
Lapin, B.A. and AL.: The monkey as an object of biomedical experiments (Sukhumi 1963).
Mann, WA., Welzel, G., and Kinter, L.B.: Determination of resting blood pressure in unrestrained cynomolgus monkeys using implanted telemetric transmitters. The Toxicologist (1991) 11: 335.
Schnell, C.R. and Wood, J.M.: Measurements of blood pressure and heart rate by telemetry in conscious, unrestrained marmosets. American Journal of Physiology (Heart and Circulatory Physiology) (1993) 264 (33): H1509-H1516.
Schnell, C.R.: Measurements of blood pressure, heart rate, body temperature, ECG and activity by telemetry in conscious unrestrained marmosets. Proceedings of the fifth FELASA symposium, Welfare and science, Brighton, (1993) pp 107-111.
Authors' address:
C. R. Schnell: K 125 2.08, Klybeckstrasse 141, NOVARTIS, 4002 Basel, Switzerland. E-mail: [email protected]
R Gerber: Anthropological Institute and Museum, University of Zürich, Switzerland.
University of Zürich, Switzerland.
This article originally appeared in Primate Report 49, 61-70, 1997.
Reprinted with permission of the Editor.