Hannah M. Buchanan-Smith
Scottish Primate Research Group, Department of Psychology,
University of Stirling, Stirling, FK9 4LA, Scotland
New World primates have evolved over millions of years and have developed complex physiological, anatomical and behavioral adaptations to live in Neotropical forests (Figure 1). Caretakers should seek knowledge of the natural lifestyles of the primates in their charge, and attempt to reproduce in the captive environment the salient aspects of the natural habitats that are biologically relevant to the animals.
 The aim of this chapter is to attempt to identify some of these aspects that can be reproduced in captivity. The aspects covered are climatic considerations, cage size and complexity, diet and food acquisition, and the social environment. The theme running throughout is that primates should have some control over their environment and activities as this appears to promote well-being on both psychological and physiological levels. Issues relating to the handling of New World primates are also discussed.
The aim of this chapter is to attempt to identify some of these aspects that can be reproduced in captivity. The aspects covered are climatic considerations, cage size and complexity, diet and food acquisition, and the social environment. The theme running throughout is that primates should have some control over their environment and activities as this appears to promote well-being on both psychological and physiological levels. Issues relating to the handling of New World primates are also discussed.
The first point to note about New World primates is their diversity;each species occupies a distinct ecological niche. Therefore, no standard captive environment can be advocated, but rather the housing and husbandry should take into account both species and individual differences. Fortunately,there are field data available for those New World primates most commonly kept in laboratories. In designing their captive environments, both the needs of the animals and the needs of the caretakers must be taken into consideration.
Climatic considerations
While there are guidelines for indoor colonies to maintain temperatures and humidities within the natural range, light has received very little attention. However, there is growing evidence that illumination may directly affect the monkeys' breeding success, such that they breed better in a bright than in a dim environment. In addition, illumination affects behavior; for example those in brighter conditions engage in more loco motor activity than those housed in dim lighting conditions. It is therefore recommended that all monkeys in a colony room are well illuminated and exposed not only to the same quantity, but also to the same quality of light.
Furthermore, providing different degrees of illumination within an enclosure(preferably sunshine and shade) gives the monkeys a choice of exposure.If natural lighting is not possible, rooms illuminated by artificial fluorescent lighting may be operated by automatic time switches that are adjusted seasonally to mimic the natural changes in day length in the species' habitat. Fading light in and out is beneficial so primates are not plunged in and out of lightness and darkness. The night monkey (owl monkey), the only nocturnal higher primate, obviously has different light requirements than diurnal primates.
Cage size and complexity
The enclosure may provide all the basic resources (e.g., food and water)within a comparatively small area, but the requirements of primates extend beyond these basic physiological needs. During the 1993 Berlin Workshop on the Accommodation of Laboratory Animals in Accordance with Animal Welfare Requirements, primatological experts recommended a minimum floor space of 1 square meter for one or two marmosets, with a further 0.25 square meters for each additional animal. The minimum cage height was set at 1.5m. These recommendations were made in the light of recent knowledge and clearly allow greater scope to meet the behavioral needs of the animals in terms of adequate space for exercise and room for environmental enrichment than previous guidelines for minimum cage sizes. Unfortunately, the Berlin Workshop did not make recommendations for other New World primates. However, cage dimensions as recommended by the Berlin Workshop for Old World monkeys are appropriate also for New World monkeys, to account for the animals' biological adaptation to an arboreal lifestyle in which the vertical dimension of space has paramount importance.
Where cages are stacked one above the other, monkeys housed in the upper row show more huddling and other species-typical activities than monkeys living in the lower row. Lower-row cages are unlikely to be suitable for any arboreal monkeys, because they restrict the animals to a quasi-terrestrial lifestyle to which they are not adapted. When given the choice, they will seek out high-level structures and approach the floor only with reluctance.
Appropriate cage furnishing enhances spatial complexity and allows better utilization of the vertical dimension. The type of cage furnishing, its dimension, orientation, its placement and its sanitary properties will have to differ from species to species, taking into consideration different needs and preferences. As a minimum recommendation, all cages should include branches to locomote on, shelves or perches to rest and groom upon, and a moveable structure such as a swing to provide unpredictability. A solid floor (as opposed to wire mesh) with a layer of sawdust mixed with small food items encourages foraging activities.
It is essential that cage furnishing does not interfere with routine animal husbandry procedures such as feeding, cleaning and capture of the cage occupant(s). However, this should not be at the expense of providing environmental complexity and stimulation. New World primates housed in naturalistic environments in zoos are no more likely to succumb to disease than those in sterile laboratory environments. The issue of over-cleaning is particularly relevant to marmosets, tamarins and night monkeys as scent marking and olfactory communication are highly developed in these species.Therefore, it is worth considering cleaning only half the cage at any onetime to ensure continual olfactory cage familiarity.
 Introducing novel objects can occupy animals for long periods, and decrease the occurrence of behavioral disorders. To overcome the problem of habituation,objects should be changed regularly if they are to remain effective. Almost any object that is safe can be used. Considerations for safety include that the object does not shatter or have sharp edges, does not contain staples or other removable metallic parts; paper or cardboard should be chlorine free. The objects should be large enough so they cannot be swallowed, but small and light weight enough for the monkeys to handle and manipulate. Environmental enrichment objects should be inexpensive and simple to prepare, clean, maintain and replace. Examples of appropriate objects include cardboard boxes, plastic tubes or containers, and balls. Objects which respond to the monkey in some way, such as being destructible or providing food (Figure 2) are particularly effective in enticing the subject to interact with them frequently and for long periods of time.
Introducing novel objects can occupy animals for long periods, and decrease the occurrence of behavioral disorders. To overcome the problem of habituation,objects should be changed regularly if they are to remain effective. Almost any object that is safe can be used. Considerations for safety include that the object does not shatter or have sharp edges, does not contain staples or other removable metallic parts; paper or cardboard should be chlorine free. The objects should be large enough so they cannot be swallowed, but small and light weight enough for the monkeys to handle and manipulate. Environmental enrichment objects should be inexpensive and simple to prepare, clean, maintain and replace. Examples of appropriate objects include cardboard boxes, plastic tubes or containers, and balls. Objects which respond to the monkey in some way, such as being destructible or providing food (Figure 2) are particularly effective in enticing the subject to interact with them frequently and for long periods of time.
Diet and foraging
Primates have a very diverse diet in the wild, and it is important to consider both nutritional content and variability of foods given in captivity. In the wild, New World primates may spend up to 50-60% of their time engaged in food-related activities throughout the day. They display a variety of specialized foraging adaptations which they cannot express in captivity if food is freely accessible. The inability to express such activities creates a void which may be filled with behavioral disorders such as stereotypies. Distributing food several times throughout the day,and making it hard to find or obtain, will more closely resemble natural conditions, and decrease the chances that the animals develop such problems.
 In social groups, key resources, such as food and water should be offered in multiple locations to minimize the risk of one monkey monopolizing them. Food dishes should be placed well above the floor level.
In social groups, key resources, such as food and water should be offered in multiple locations to minimize the risk of one monkey monopolizing them. Food dishes should be placed well above the floor level.
There is a growing number of studies which have described and tested enrichment devices designed specifically to elicit natural foraging adaptations. For example, marmosets have specialized dentition for gouging gum-wells in which exudates accumulate (Figure 3). An artificial gum tree is an inexpensive way of promoting this natural activity (Figure 4). The gum tree consists of a stack of cylindrical blocks of hardwood dowel, approximately 5 cm in diameter and cut into 7 cm lengths. Each block has a central hole through which a threaded brass rod is inserted and secured at either end with a washer and wing nut.
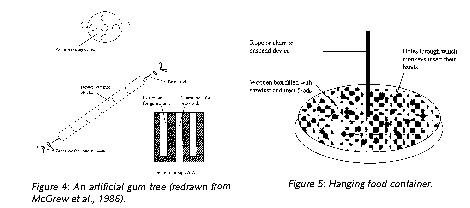
Each wooden block has four reservoirs into which gum arabic is syringed (gum arabic is used widely in the human food industry and is therefore readily available). The blocks are tightly stacked so the marmosets have to gnaw to access the gum. In colonies where artificial gum trees may be impractical, simply offering untreated wood for gnawing is better than nothing at all.
Other simple methods to promote species-typical foraging activities include scattering food on the floor covering (or mixing them in sawdust in purpose-built foraging boxes) so the monkeys have to search for it. A more sophisticated method of increasing foraging is to suspend a foraging container from the roof of the enclosure (Figure 5). The monkeys insert their hands through the holes in search of food items. Suspending the device challenges the monkeys to engage in complex motor coordination and provides a degree of unpredictability, especially if two animals are extracting food from it simultaneously. The size of the device depends upon which species it is designed for. Another technique is to spear whole fruits and vegetables on bamboo canes and suspend them from a branch.
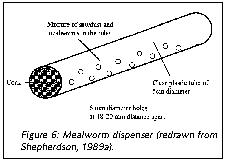 For New World monkeys for whom insects form a substantial part of the natural diet (e.g., marmosets, tamarins, squirrel monkeys), simple cricket or mealworm dispensers (Figure 6) offer inexpensive ways to provide extra variability in the environment. In these feeders, mealworms and crickets appear at unpredictable intervals, and necessitate an adaptive response from the monkey to find, capture and retrieve them. Capuchin monkeys will readily use tools to retrieve and process food. Providing them with appropriate-sized sticks and stones gives them an opportunity to utilize their skills. They will use stones to crack nuts and other hard-shelled food items, and will use the sticks to rake food in or poke it out of a container (Figures 7 and 8).
For New World monkeys for whom insects form a substantial part of the natural diet (e.g., marmosets, tamarins, squirrel monkeys), simple cricket or mealworm dispensers (Figure 6) offer inexpensive ways to provide extra variability in the environment. In these feeders, mealworms and crickets appear at unpredictable intervals, and necessitate an adaptive response from the monkey to find, capture and retrieve them. Capuchin monkeys will readily use tools to retrieve and process food. Providing them with appropriate-sized sticks and stones gives them an opportunity to utilize their skills. They will use stones to crack nuts and other hard-shelled food items, and will use the sticks to rake food in or poke it out of a container (Figures 7 and 8).
Social environment
Companionship is undoubtedly most important for captive New World primate. All species have strong social needs; they have evolved complex modes of olfactory, vocal, tactile and visual communication. Furthermore, as much of the behavioral repertoire is learned from other conspecifics, appropriate social housing is of paramount importance for the development of young animals.
 The type of social grouping in the wild should be used as a guide for social grouping in the laboratory in order to ensure optimal group compatibility and reproduction.
The type of social grouping in the wild should be used as a guide for social grouping in the laboratory in order to ensure optimal group compatibility and reproduction.
When forming new groups, great care must be taken that all monkeys are compatible. When establishing new pairs, it is advisable to allow the partners visual, auditory and olfactory contact for several days prior to pair formation. If no, or only few, non-serious aggressive disputes occur during this familiarization period, physical contact can be allowed. It is preferable to form new pairs in an environment with which both members are equally familiar rather than transferring one of them into the other's home enclosure. The latter situation may trigger territorial aggression.
When introducing a new individual to an already established social group, visual contact is again recommended as a first step. This is followed by pairing the new animal with each individual member of the group in a "neutral" environment for extended periods of time to allow the new member to form affiliative social relationships with all group members before final group introduction. The new group or pair should be closely observed during the initial stages, and at regular intervals thereafter to determine compatibility. Grooming, huddling, mating and play are behaviors indicative of compatibility, whilst aggression or signs of withdrawal, fear and inactivity suggest incompatibility.Attention should also be focused on possible loss of weight and deterioration of body condition possibly resulting from social incompatibility.
In established social groups, matured progeny should be removed at an appropriate time to avoid fighting and inbreeding. For marmosets and tamarins, however, it is advisable not to remove male or female offspring until they have had experience with two sets of rearing episodes. This is a safeguard that they will become good parents themselves. Animals who are removed from their parental group should be transferred to new social housing arrangements.
 New World primate groups are often kept in visual and tactile isolation from each other in the laboratory, although olfactory and auditory contact is usually possible. It is known that neighboring groups of New World primates meet regularly in the wild. There are a number of ways this can be mimicked in captivity. For example, a small "peep hole" can be made between adjacent cages to allow individuals to look at their neighbors who don't know that they are being watched. Cages can be easily interconnected with wire mesh tunnels or ducting allowing the monkeys to travel back and forth from their home enclosure to adjacent cages where they may encounter members of the other group in a non-physical contact situation (Figure 9). In colony rooms where several groups are housed, providing turrets of wire mesh at the top of the home cage allows monkeys to decide if and when they wish to be in visual contact with neighboring groups. This gives them some control over their social environment.
New World primate groups are often kept in visual and tactile isolation from each other in the laboratory, although olfactory and auditory contact is usually possible. It is known that neighboring groups of New World primates meet regularly in the wild. There are a number of ways this can be mimicked in captivity. For example, a small "peep hole" can be made between adjacent cages to allow individuals to look at their neighbors who don't know that they are being watched. Cages can be easily interconnected with wire mesh tunnels or ducting allowing the monkeys to travel back and forth from their home enclosure to adjacent cages where they may encounter members of the other group in a non-physical contact situation (Figure 9). In colony rooms where several groups are housed, providing turrets of wire mesh at the top of the home cage allows monkeys to decide if and when they wish to be in visual contact with neighboring groups. This gives them some control over their social environment.
Caretakers and Monitoring
The importance of the relationship between monkeys and their caretakers cannot be overemphasized. It is the caretaker who monitors the behavior on a day-to-day basis, checks that the monkeys are in good physical condition,that they are not being peripheralized by other members of the social group, that they are eating etc. It is important when enriching an enclosure, that data are collected to guarantee that any environmental manipulation has its desired effect.
Caretakers are often the most suitable people to collect such data. Effective assessment requires careful selection of appropriate measures.
The standard protocol is to collect "baseline" data to determine the behavior of the monkeys before any environmental manipulation occurs. The change is then made and the same behaviors are recorded, possibly with the addition of other behaviors which relate directly to the change. Certain behaviors such as foraging, resting, locomotion, play, grooming or huddling may be considered important in any data collection session as they occur frequently and provide a general indicator of the monkeys' behavioral health. Assessing agonistic and affiliative behavior helps to determine the relationships of group members, which in turn can be very valuable to know during husbandry procedures. The frequencies of behavioral disorders such as stereotyped movement or coprophagy (eating feces) should also be recorded. Other activities may be quite species-specific, or task-specific. For instance, forty percent of squirrel monkey locomotion is accomplished by leaping. An increase in the occurrence of this behavior could be taken as an indicator of the success of a project which changed the enclosure or furnishings. Exploratory and manipulatory behavior towards novel objects would be appropriate measures of assessing how much attention the monkeys pay to them, and thus how effective they are in occupying the monkeys' time in a species-appropriate manner. Collecting observational data at regular intervals is a quantitative measure of the animals' behavioral responses to changes in their environment. This is especially important, as an alteration in a monkey's behavior may be gradual and go unnoticed unless it is clearly quantified by such records.
Handling
Catching monkeys in nets can cause them injuries as they engage in vigorous avoidance responses. It is not advisable to capture a monkey who carries an infant, as the infant may be bitten accidentally by the panicking adult. Habituating the monkeys to enter a handling cage and gently capturing them by hand is preferable. This can be achieved by repeatedly rewarding a subject with treat foods in an open handling cage on several days prior to actual capture. Moving marmosets and tamarins between enclosures can easily bed one through flexible ducting or through wire tunnels, thus obviating the need for capture.
In research protocols which require handling procedures, the monkey should be positively reinforced. For urine collection, subjects can be readily trained to cooperate, making the need for capture or removal from the social group unnecessary.
The quality of the captive environment of New World primates not only affects welfare, but it also affects the outcome of research, as housing and husbandry conditions have a direct impact on experimental results. Laboratory housing that adequately caters for the species-characteristic needs of the animals and minimizes stress is important on both of these counts.
Acknowledgments
Figure 3 is reproduced from Humane Innovations and Alternatives with kind permission from the editor. James Anderson kindly provided Figures 7 and 8. I thank Viktor Reinhardt, Cathy Liss and John Barnes for their critical comments on this manuscript.
References
Anzenberger G. Gossweiler H 1993. How to obtain individual urine samples from undisturbed marmoset families. American Journal of Primatology 31, 223-230.
Coimbra-Filho AF, Mittermeier RA 1988 (eds). Ecology and Behavior of Neotropical Primates vol 1, Washington DC, World Wildlife Fund.
Boinski S. Noon C, Stans S. Samudo R. Sammarco P. Hayes A 1994. The behavioral profile and environmental enrichment of a squirrel monkey colony.Laboratory Primate Newsletter 33 (4), 1-4.
Box HO, Rohrhuber B 1993. Differences in behaviour among adult male,female pairs of cotton-top tamarins (Saguinus Oedipus) in different conditions of housing. Animal Technology 44, 19-30.
Buchanan-Smith HM 1991. A field study on the red-bellied tamarins (Saguinuslabiatus) in Bolivia. International Journal of Primatology 12,259-276.
Garber PA 1984. Use of habitat and positional behavior in a Neotropicalprimate (Saguinus Oedipus). In: Adaptations for foraging in non human primates (Rodman P. Cant J. eds). New York, Columbia University Press,pp 112- 133.
Guidelines on the care of laboratory animals and their use in scientific purposes. Part 1: Housing and care. The Royal Society and the Universities Federation for Animal Welfare. London, 1987.
Heger W. Merker HJ, Neubert D 1986. Low light intensity decreases the fertility of Callithrix jacchus. Primate Report 14, 260.
McGrew WC, Brennan JA, Russell J 1986. An artificial "gum-tree"for marmosets (Callithrix j. jacchus). Zoo Biology 5, 45-50.
Mittermeier RA, Rylands AB, Coimbra-Filho AF, da Fonseca GAB 1988. (eds).Ecology and Behavior of Neotropical Primates vol 2, Washington DC,World Wildlife Fund.
Moore K, Cleland J. McGrew WC 1991. Visual encounters between families of cotton-top tamarins, Saguinus Oedipus. Primates 32, 23-33.
O'Donoghue PN 1994 (ed) The accommodation of laboratory animals in accordancewith animal welfare requirements. Proceedings of an international workshop held at the Bundesgesundheitsamt, Berlin 17- 19 May, 1993. Bundesminsteriumfur Ernahrung, Landwirtschaft und Forsten, Bonn.
Price EC, McGrew WC 1990. Cotton-top tamarins (Saguinus (o) oedipus)in a semi naturalistic captive colony. American Journal of Primatology 20, 1- 12 .
Rylands, AB 1993. (ed) Marmosets and Tamarins: Systematics, Behaviour and Ecology.
Schoenfeld D 1989. Effects of environmental improvement on the social behavior of marmosets (Callithrix jacchus). American Journal of Primatology Suppl 1, 45-51.
Scott L 1991. Environmental enrichment for single housed common marmosets. In: Primate responses to environmental change (Box HO, ed). London,Chapman and Hall, pp 265-274.
Sheperdson D 1989a. Environmental enrichment in zoos: 2. RATEL 16, 68-73.
Sheperdson D 1989b. Environmental enrichment-measuring the behaviour of animals. RATEL 16, 134- 139.
Snowdon CT, Savage A, McConnell PB 1985. A breeding colony of cotton-top tamarins (Saguinus oedipus). Laboratory Animal Science 35, 477-480.