Arnold S. Chamove, James R. Anderson, Susan C. Morgan-Jones, and Susan P. Jones
Sixty-seven animals from eight primate species were used to assess improved husbandry techniques. The presence of woodchips as a direct-contact litter decreased inactivity and fighting, and increased time spent on the ground. Placing food in the deep litter led to further behavioral improvement. The use of frozen foods improved food distribution and reduced fighting in most situations, especially when it was buried in the litter. With time, the litter became increasingly inhibitory to bacteria. The results suggest that inexpensive ways of increasing environmental complexity are effective in improving housing for primates.
Introduction
A desirable objective in the management of captive animals is the creation of an environment adequate for the animals' physical and emotional needs. This is especially true for nonhuman primates in whom social, physiological, an intellectual patholologies result when important environmental considerations are neglected (McGrew, 1981). Environmental enrichment can be achieved by providing electrical and mechanical manipulanda (e.g., Chamove, in prep.; Markowitz and Woodworth,1978; Murphy, 1976), or appropriate social stimulation (Chamove, 1973), or by attempting to approximate a more natural environment, for example by providing the animals with a deep-litter substrate on floors that were bare (Chamove and Anderson, 1979). The present article reports the results of the three studies concerned with two techniques of enhancing captive conditions for primates. Two studies examined the suitability of woodchips as a deep litter for various primate species. The third study also evaluated the effects of freezing fruit on its distribution and on aggressive behavior during feeding in a macaque group.
Study 1
A previous paper (Chamove and Anderson, 1979) suggested that litter was an effective floor covering for captive macaque groups. The rationale for its use was as follows: If an animal in its natural environment spends a substantial amount of time exhibiting a particular type of behavior, e.g., searching for food, while the animal in captivity is prevented from engaging in similar types of activity, the distortion in the animal's usual pattern of activity might be stressful for the animal, leading to abnormal behaviors (Dawkins, 1980; Hediger, 1968; Meyer-Holzapfel, 1968). In captivity, food is usually presented once or twice per day, and it is therefore located and consumed in a short time. This contrasts with the extensive amount of time, up to 70 percent, that is spent in foraging activities in the wild (see references in Clutton-Brock, 1977; Harding and Teleki, 1981).
A second argument for the use of litter is an aesthetic one. Waste products are normally avoided by monkeys, but this is difficult when wastes are excreted onto solid floors. If monkeys avoid spending time on the floor of their cage because it is soiled, the area is being used inefficiently. Alternatively, the monkeys may be forced to spend time on a floor which they find aversive. Litter can serve to cover and absorb urine rapidly, and decompose feces. This study is an attempt to generalize the results of our previous pilot study of woodchip litter using stumptail macaques (Chamove and Anderson, 1979) to a variety of other primate species.
Method
The seven species of monkey and one prosimian that were studied were moustached guenons (Cercopithecus cephus, N = 8), vervets (C. aethiops, N = 4), ring-tailed lemurs (Lemur catta, N = 3), stumptail macaques (Macaca arctoides, N = 6), squirrel monkeys (Saimiri sciureus, N = 7), black-capped capuchins (Cebus apella, N = 7), red-bellied tamarins (Saguinus labiatus, N = 4), and common marmosets (Callithrix jacchus, N = 3). All were housed in Edinburgh Zoological Gardens, with the exception of the tamarins who were housed in a room in the Stirling University Psychology Primate Unit. The seven Edinburgh groups lived in indoor-outdoor enclosures. The outdoor areas contained dead trees and either grass or gravel on the ground. The floors of the indoor areas were of epoxy cement, and only this area was used for the study. Only the stumptails and tamarins had previous experience with woodchips on the floor.
Four conditions were studied: (1) baseline, i.e., bare floor; (2) woodchips on the floor; (3) woodchips plus grain; and (4) woodchips plus mealworms. Two days of observation were conducted under the first three conditions and 1 day under the fourth. Following the 2 days of baseline observation, new woodchips were spread on the floors to a depth of approximately 4 cm. One week later, observations were undertaken under this, the woodchip condition. On the following day, 500 g (approximately 800 cc) of mixed grain was scattered and raked into the woodchips, and 30 minutes later the group was tested (see below for the testing methodology). This procedure was repeated the following day, using one-third of this amount of grain. These 2 days constitute thewoodchip + grain condition. The grain mixture contained primarily millet seeds, with a small amount of peanuts, sunflower seeds, dried currants, wheat, and kibbled corn. The following day, five mealworms per animal were scattered onto the litter, and 30 minutes later the group was observed in this woodchip + mealworm condition.
Each test involved one experimenter monitoring the group for 20 minutes between 2 and 4 p.m. A metronome sounded every 10 seconds, and any behavior occurring during each interval was noted once. Threats, rough grabbing, and biting were recorded as aggression; grimaces, cowering, and fleeing were scored as fear. Stereotyped movements, bizarre postures, and self-aggression constituted "abnormal" behaviors. Affiliative behavior involved grooming or huddling with another animal. Foraging was defined as manipulating the woodchips and intermittently transferring items found in the woodchips to the mouth. All scores were converted to a percentage of the intervals during which the subject was visible, i.e., indoors. The data were analyzed using analyses of covariance. The percentage of time each subject was observed on the ground on the first 2 control days, the bare condition, was used to obtain a measure or arboreality, which was then used as a covariate (see Table 1).
TABLE 1. Time on the ground and agonistic behavior in eight species in different conditions
| Species | N | Time on ground in bare condition (%) |
Time on ground in most effective condition (%) |
Time exhibiting agonistic behavior (%) |
|
|---|---|---|---|---|---|
|
|
|
||||
| Guenon Vervet Lemur Stumptail Squirrel Capuchin Tamarin Marmoset |
4 3 6 7 7 4 3 |
17 9 8 5 1 2 0 |
26* 87 80 13* 28 14 11 |
.11 .14 .63 .20 .13 .52 .40 |
.02 .10 .18 .01 .14 .10 .06 |
Three analyses of covariance were performed. All included species (N = 8) and condition (N = 4) as factors. In addition, percentage of time spent inactive or asleep was used as a repeated measure in one analysis, as were "negative" behaviors, i.e. aggression, fear, and abnormal activities, while "positive" behaviors, i.e., play and affiliation, were employed in the second analysis. The third analysis used percentage of time on the floor, percentage of time engaged in foraging, and time spent outside as repeated measures. Alpha was set at .05, and all reported differences are significant beyond this level unless specifically stated otherwise. The least Significant Difference (LSD) method was used to further evaluate significant effects.
Results
The results from all three analyses suggested that the addition of woodchip litter altered behavior. Surprisingly, the covariate had little effect: its largest beta estimate was only 0.20 for the analysis of foraging, indicating that the effect of the woodchip litter was not related to the degree of arboreality of the species. The forage analysis (Fig. 1) revealed two interesting effects (condition X behavior, and species X condition X behavior, both P< .001): (1) All species spent more time on the ground when it was covered with woodchips than when it was bare, and (2) when grain was incorporated into the litter, a further increase was noted. Since the foraging scores were very similar to the scores for the time spent on the ground, only the latter are plotted.
The social behavior analysis showed a significant condition X behavior effect (P< .005), and a significant species X condition X behavior interaction (P < .05). The positive and negative behavior scores are plotted in Fig. 1. Plots of the observed frequency of the two negative behaviors were parallel for the four sets of conditions, but this was not true of the two positive behaviors.
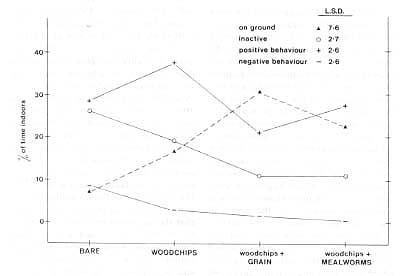
Positive = affiliation + play, negative = agonistic + abnormal. The Fisher's LSD values are for the condition X behavior interaction.
With woodchips, the relative proportion of affiliative behavior making up the positive category decreased as the environment provided was made more interesting; play was 3 times more frequent than affiliation in the bare condition, 5 times more frequent in the woodchips-only condition, and 8 times more frequent in the woodchips + food conditions. With woodchips, the subjects showed less negative and more positive behavior, in comparison with the bare condition. Grain added to the litter reduced the level of positive behavior, probably because of its distracting effects. The activity analysis showed significant effects of species X condition, and condition X behavior (both P < .001). Because sleep rarely occurred, only percentage of time spent inactive is plotted in Fig. 1. The provision of woodchips decreased inactivity.
These results suggest that the mere presence of litter leads to positive behavioral changes, even after the novelty effects of its presence have passed. All species were less inactive; all except squirrel and vervet monkeys showed more play; all except capuchins engaged in a lower frequency of abnormal and agonistic behaviors; and all except marmosets spent more time on the ground foraging. The addition of grain or mealworms to the woodchips greatly increased the time spent on the ground, reduced inactivity, reduced play and affiliative behaviors, and tended to reduce aggression even further than with litter alone. Grain was particularly attractive to the stumptail macaques, lemurs, and vervet monkeys, while mealworms were particularly attractive to the tamarins and moustached guenons. This effect is shown in Table 1, which gives the condition that produced the greatest amount of time on the ground for each species.
Study 2
Study 1 confirmed and extended the finding that the use of woodchip litter with captive monkeys leads to positive behavioral changes. Furthermore, in our previous report the chips were shown to be inexpensive; after 6 weeks, odor was less than with bare floors, and the animals and walls appeared cleaner when woodchips were provided than when there was no floor covering but daily cleaning was performed (Chamove and Anderson, 1979).
One criticism of using litter with monkeys focuses on the danger of a buildup of disease, with the implicit assumption (Department of Health, Education, and Welfare, 1972) that the longer the litter is left down, the greater the danger. However, evidence from research on poultry litter suggests precisely the opposite, by demonstrating that mature litter is inhibitory to many disease organisms as well as to yeasts and molds (Fanelli, 1970; Snoyenbos, 1967; Tucker, 1967; reviewed in: Anon. 1978; Botts et al., 1952; Duff et al., 1973; Olesiuk et al., 1971).
Chicks reared on old litter have lower mortality and grow more rapidly than controls. In addition, their eggs show increased hatchability (Botts et al., 1952). The mere presence of old or new litter was shown by Duff et al. (1973) to eliminate the spread of salmonella among experimentally infected chicks. Although salmonellas survive for 3 to 4 weeks in feces (Berkowitz et al., 1974), in used litter they are substantially destroyed within 3 to 5 days (Olesiuk et al., 1971). The mechanism of salmonellacidal action is unclear, but there are suggestions that the increased moisture content (up to 20 percent), coupled with the high ammonia concentration and resulting alkalinity, are the critical factors (Turnbull and Snoyenbos, 1973). Study 2 assessed the potential for the spread of disease in litter used with macaque monkeys.
Method
Twenty-five stumptail macaques {Macaca arctoides), with a mean weight of 6.5 kg, were housed in an area composed of an indoor colony room and two outside areas of 33 sq m and 20 sq m respectively {described and illustrated in Chamove, 1981). All cages were interconnecting, and the animals were free to roam throughout the three areas. The outside pens were covered with mesh and partly covered with clear plastic. The floor area of each of the outside pens was covered with three 40-kg bales of woodchips. Twelve samples were taken from weeks 0 to 8 during July and August 1981. The samples were collected randomly from five different areas of a pen and mixed. Figure 2 illustrates members of a group of 25 stumptail macaques foraging through woodchips in an outside pen. Chips are covering only half of the pen floor.

Microbiological Analysis. One gram of the litter was taken, and serial dilutions were prepared using 1/4-strength Ringer solution (Oxoid no. BR 52) as the diluent. Appropriate dilutions were plated on nutrient agar (Oxoid no. CH 3) using standard techniques (Ministry of Agriculture, Fisheries and Food, 1968) Coli-aerogenes bacteria were counted at 30ºC (Meynell and Meynell, 1970), using MacCartney broth (Oxoid no. CH 5a). All tubes showing acid and gas production after 48 hours were subcultured into duplicate tubes of fresh media; one tube was incubated at 37 ± 1ºC, and the other at 44 ± 0.25ºC.
Because salmonella is such a common and serious disease-producing organism in monkeys (Chamove et al., 1979), the inhibiting effect of the litter on Salmonella typhimurium was assessed by inoculating approximately 103 organisms into 1 g of litter in a MacCartney bottle and then shaking and incubating it at 22ºC for 48 hours. The numbers of salmonella organisms in the litter after storage were estimated using the method described by Morgan-Jones (1982).
Results
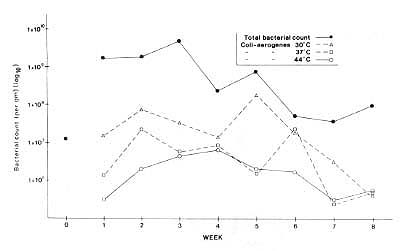
Correlations of times (age of litter, expressed in weeks) with bacterial counts ranged from –.41 for the total count to –.60 for salmonella, and between –.70 and –.76 for the three coliforms. Although pH and percentage of dry matter correlated highly with week number (r = + .65 and –.59, respectively) and also with one another (r = –.60), the correlation between pH and week number did not seem to be caused by moisture content, since partialling out percentage dry matter did not substantially reduce the correlation (r = + .50).
Similarly, with one exception the correlation of bacterial inhibition with week number was not accounted for by either moisture content or pH of the litter. Partialling out the variance due to percentage of dry matter reduced the bacterial correlation with week number by only .04, on average; partialling out pH reduced it by only .03, except for the 37ºC test (.14) and the total count, where it actually increased by .25.
It is clear from Fig. 3 that the total bacteria count decreased over the weeks. This was also true for coliforms isolated at 30ºC, which include coli-aerogenes of both animal and nonanimal origin; 37ºC, which reflect coliform bacteria of fecal origin; and 44ºC, which reflect coliforms of very recent fecal origin. The survival tests for inoculated salmonella showed a similar pattern of reduced survival over the weeks. The numbers of salmonella rose from 2.9 x 104 per 9 in week 0 to a maximum of 2.4 x 106 in week 1, then gradually declined to a minimum of 4.3 x 101 by week 8 (weeks 2 to 7: 2.4 x 104, 3.3 x 104,3.3 x 104, 4.6 x 103, 1.5 x 104,1.1 x 103, 2.3 x 102). It is of interest here that the monkey litter was as inhibiting to salmonellas as is poultry litter (Morgan-Jones unpublished data).
These results show that the use of litter will not increase the risk of bacterial disease transmission and in fact appreciably reduces that risk. We have observed that after a period of about 12 weeks the monkeys spend less time on the litter and are less interested in searching through it. This behavioral criterion is useful in the scheduling of litter changes; we have decided that renewal every 4 to 6 weeks is optimal at our population densities.
Study 3
Fresh fruit and vegetables are usually given to captive monkeys to relieve the boredom of standardized diets. Two problems that often occur when feeding group-housed animals are: (1) the dominant animals are able to expropriate a disproportionate amount of the food, and (2) the food is eaten too quickly. We have observed that feeding solidly frozen fruits and vegetables to monkeys leads to better distribution and longer feeding times (Chamove, 1981), and have been using this method for the past 7 years with no ill effects. Study 3 was carried out to quantify and verify our earlier observations.
Methods
The Stirling colony group of 25 stumptail macaques was used. Their ages ranged from 6 months to 8 years, with a mode of about 2 years. Four experimental comparisons were made. (1) To assess the influence of incentive, three foods were offered in decreasing order of preference—banana, apple, and carrot. (2) T o assess the effect of manner of distribution, food was either massed in two piles or distributed evenly over the floor area. (3) To assess the effects of inter-animal visibility, the food was either distributed in the outside area where all subjects could see one another when feeding, or distributed over the same area inside where four opaque dividers with openings restricted visual contact among subjects. (4) To assess the effects of visibility of food, the food was either distributed on a bare area of the outside floor as above or buried under woodchips in the same area.
In all conditions two tests were run, one using fresh food, the other using from food. In all tests except experiment 1 the food used was apple. In each test the total weight of the food, cut into 45 pieces, was 1.25 kg.
Four measures were recorded on nine selected animals. The measures were (1) the number of food items eaten, i.e., picked up and more than one bite taken from it; (2) the number of items eaten plus sampled, i.e., dropped after only one bite was taken from it; (3) the number of agonistic interactions; and (4) the time that elapsed until all of the food had been consumed.
The analysis used analyses of variance with subjects divided into dominant (N = 2) and subordinate (N = 7) subgroups. All results reported below are significant beyond the .05 level unless specifically stated otherwise.
Results
Figure 3 illustrates the major significant differences observed. Under the condition in which food was distributed, freezing the food reduced aggression by a factor of 3 but had only a slight positive effect on distribution of food among the animals. In general, as the possibility of the dominant monkeys seeing and controlling all the food items decreased (under the conditions displayed from left to right in Fig. 4), the amount consumed by the dominants decreased, the amount eaten by the subordinates increased, and aggression was reduced. This effect was accentuated when the food was frozen.
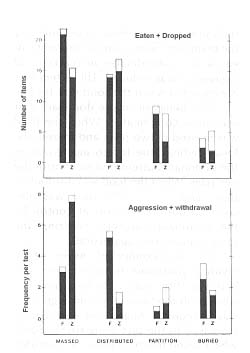
The behavior of the dominant pair was more complicated. When the food was massed in two piles and frozen, the long feeding time led to aggression as the dominants attempted to control the two piles. When the food was distributed, fresh, and visible, aggression was also common due to attempts at control by the dominant subjects. Freezing the food reduced this aggression.
The test conducted inside, where dividing partitions restricted inter-animal visibility, was over in 2 minutes when fresh food was used, and aggression was infrequent. Aggression was slightly increased in the test using frozen food, which lasted much longer —24.3 minutes. Corresponding durations from the tests done outside were 6.4 and 19.0 minutes. To provide some perspective on these values, an adult stumptail eats an apple in about 1.8 minutes and a banana in about 0.9 minutes. A frozen apple or banana takes about six times as long to eat.
In the tests involving three types of distributed food, the dominants ate relatively more of the two preferred foods when it was offered fresh than when it was frozen, but not of the carrot. Aggression by the dominant monkeys was over four times greater for banana and apple when these were fresh than when they were frozen, but aggression was roughly equal (when fresh) and much lower (frozen) for the carrot.
Discussion
The results of the present studies clearly show that there are advantages to using woodchips as a substrate for monkeys. These data thus support the conclusions reached in a previous study with stumptail macaques (Chamove and Anderson, 1979). I n the present study, using more species, aggressive behavior was reduced by a factor of 3 with woodchips and by almost 10 times with grain or mealworms added to the litter. All negative behavior decreased by a factor of over 5 when food was added to the woodchips. Time spent on the ground almost doubled with woodchips, and more than doubled when food items were added to it. These effects occur in monkeys of various ages. Figure 5 illustrates a group of stumptail monkeys foraging through woodwool, another type of litter we are evaluating. We have observed that it does not "pack" in the same way as woodchips do, and may therefore be left down longer.

In addition to searching through the two types of litter, juveniles also engage in playful gymnastics in them, more so than on a bare floor, and more on woodwool than on woodchips.
In addition, there is no evidence that using woodchips presents a health hazard. As the litter matures, the woodchips become increasingly more inhibitory to bacterial survival. This self-sterilizing action makes it likely that the mere presence of an absorbent litter greatly reduces the probability of disease spread due to fecal contamination.
The freezing of food also has advantages in certain situations, leading to improved distribution and less fighting. This is particularly true when the dominant animals cannot "control" the food sites. Distribution of the food per se in a small enclosure may not reduce aggression, because the dominant animals may try to monopolize most of the food that they can see. One method of reducing the dominant animals' ability to control the food—burying it—resulted in improved distribution and prolonged feeding times. We regularly bury small food and non-food items in the woodchips, which the monkeys seem to enjoy discovering.
In conclusion, we recommend deep litter as one technique of enhancing conditions for captive primates. It has real potential for promoting good health and induces positive kinds of behavior among species that invest a great deal of time and energy in foraging in their natural environment.
Acknowledgments
The authors wish to thank M. Stevenson for help and permission at Edinburgh Zoo, and Miss A. Coles for technical assistance with the microbiological analysis.
References
Anonymous (1978) Microbiology associated with hatching, rearing and processing of poultry. In: Ann Rep Edin School Agr, pp. 66-67.
Berkowitz, J .H., Kraft, D.J. and Finstein, M.S. (1974) Persistence of salmonellae in poultry excreta. J Environ Qual 3: 158-161.
Botts, C. W., Ferguson, L.C., Birkeland, J. M., and Winter, A.R. (1952) Influence of litter on the control of salmonella infections in chicks. Am J Vet Res 13: 562-565.
Chamove, A.S. (1973) Varying infant rhesus social housing. J Inst Anim Tech 24:5-15.
Chamove, A.S. (1981) Establishment of a breeding colony of stumptailed monkeys. Lab Anim 15:251-259.
Chamove, A.S. and Anderson, J.R. (1979) Woodchip litter in macaque groups. J Inst Anim Tech 30:69-72.
Chamove, A.S., Cameron, G. and Nash, V. J. (1979) Primate disease and breeding rates. Lab Anim 13:313-316.
Clutton-Brock, T.H. (1977) Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes. Academic Press, London.
Dawkins, M.S. (1980) Animal Suffering. Chapman-Hall, London.
Department of Health, Education and Welfare (1972) Guide for the Care and Use of Laboratory Animals. Department of Health, Education and Welfare, Publication No. (NIH) 73-77, Washington, DC.
Duff, R.H., Ross, J.G. and Brown, D.D. (1973) The influence of litter on Salmonella typhimurium infection in poultry. Avian Pathol 2:263-268.
Fanelli, M.J. (1970) Preliminary studies on persistence of salmonellae in poultry litter. Avian dis 14:131-141.
Harding, R.S.O. and Teleki, G., eds. (1981) Omnivorous Primates. Columbia University Press, New York, NY.
Hediger, H. (1968) The Psychology and Behavior of Animals in Zoos and Circuses. Dover, New York, NY.
Ministry of Agriculture, Fisheries and Food (1968) Bacteriological Techniques for Dairy Purposes, Technical Bulletin No.17. HMSO, London.
Markowitz, H. and Woodworth, G. (1978) Experimental analysis and control of group behavior. In: Markowitz, H. and Stevens, V .J ., eds., Behavior of Captive Wild Animals. Nelson-Hall, Chicago, IL.
McGrew, W.C. (1981) Social and cognitive capabilities of nonhuman primates: lessons from the wild to captivity. Int J Study Anim Prob 2:138-149.
Meyer-Holzapfel, M. (1968) Abnormal behavior in zoo animals. In: Fox, M.W., ed., Abnormal Behavior in Animals. W. B. Saunders, Philadelphia.
Meynell, G.G. and Meynell, E. (1970) Theory and Practice in Experimental Bacteriology. Cambridge University Press, London, U.K.
Morgan-Jones, S.C. (1982) A method of enumerating salmonellas in poultry environments. In: Corry, J.K.L., Roberts, D. and Smith, D.G., eds., Methods for the Isolation and Identification of Food Poisoning Organisms, Society of Applied Bacteriology Technical series No. 17. Academic Press, London (in press).
Murphy, D.E. (1976) Enrichment and occupational devices for orangutans and chimpanzees. Int Zoo News 23: 24-26.
Olesiuk, O.M., Snoyenbos, G.H. and Smyser, C.F. (1971) Inhibitory effects of used litter on Salmonella typhimurium transmission in the chicken. Avian Dis 15:118-124.
Snoeyenbos, G.H. (1967) An epidemiological study of salmonellosis in chickens, Avian Dis 11 :653-667.
Tucker, J.F. (1967) Survival of salmonellae in built-up litter for housing of rearing and laying fowls. Br Vet J 123:92-103.
Turnbull, P.C.B. and Snoyenbos, G.H. (1973) The roles of ammonia, water activity, and pH in the salmonellacidal effect of long-used poultry litter. Poult Sci 12:72-86.
This article was reproduced with permission of the senior author. It was originally published in The International Journal for the Study of Animal Problems 3(4) 1982.