J R Anderson, A Rortais and S Guilletemein
Laboratoire de Psychophysiologie (CNRS URA 1295), Université Louis Pasteur
7 rue de l'Université, 67000 Strasbourg, France
Abstract
In order to assess the environmental enrichment value of a small swimming pool for captive juvenile rhesus macaques (Macaca. mulatta), observations of social and individual behaviours were made during baseline and experimental (pool) conditions. When the pool was available there was less social grooming and cage manipulation, and more play. Most of the monkeys engaged in diving and underwater swimming. The presence of pieces of banana at the bottom of the pool reduced these water-related activities, whereas when raisins were spread along the bottom or when there was no food in the water, there was more diving and less aggression. Certain effects tended to vary with dominance status, but individual differences appeared more important than social status in determining reactions to the water. The provision of a small swimming pool for captive macaques is an effective contribution to improving their welfare.
Keywords: aggression, animal welfare, dominance, environmental enrichment, play, rhesus macaques, swimming
Introduction
Swimming has been reported to occur in wild populations of many species of Old World monkeys (e.g. Roonwal & Mohnot 1977). Usually, swimming is functional, e.g. necessary for reaching sleeping or foraging sites (long-tailed macaques: Fittinghoff & Lindburg 1980; chacma baboons: Hamilton 1982; proboscis monkeys: Yeager 1991) or to escape from predators (talapoin monkeys: Gautier-Hion 1973). However, recreational swimming has been described in certain free-ranging populations of macaques (Kawai 1965; Lindburg 1971; Berman 1977). Some zoos and wildlife parks provide facilities to encourage swimming in macaques (e.g. Tokyo Zoo in Japan, Monkey Jungle in Florida, Zoo de Labenne in France), and swimming has recently been described as a feasible recreational activity for laboratory-housed macaques (Gilbert & Wrenshall 1989; Anderson et al 1992). Although captive macaques may appear highly motivated to dive into water and swim, there are no published comparisons of behaviour when deep water is absent and when it is available (but Parks & Novak 1993 report on the effects of providing shallow water for captive rhesus monkeys). In the study by Anderson et al (1992), out of a group of 17 juvenile female rhesus macaques, 15 were observed to repeatedly dive into a small swimming pool and engage in underwater swimming. As well as appearing to enjoy entering the water, the monkeys also seemed to engage in more overall play when the pool was available, but quantitative observations were not conducted on this aspect. Thus, until now there exist no formal data on the apparent enrichment effects of the availability of a swimming pool for captive monkeys. An appropriate analysis would involve the comparison of behaviour in the presence and the absence of the pool, with each animal serving as its own control (Poole 1988).
In the present study, we assessed the effects of the presence of a small swimming pool on the social and individual behaviours of the group of juvenile rhesus macaques studied by Anderson et al(1992). If the emergence of play is facilitated by low-stress environments (Smith 1978; Baldwin 1986), and if play increases when the pool is available, then the enrichment value of the pool would become clearer. In order to extend assessment of the pool as an enrichment, we included four different pool conditions: one in which there was no food in the water, and three in which food was present. Since we had previously found that the presence of a submerged box containing food led to some social tension in the group, it was predicted that the absence of food-related competition in the no-food condition would result in less aggression and more diving and underwater swimming than the pool-plus-food conditions. Dominance was included as an independent variable, as this factor has been found to influence responses to enrichment procedures in other studies (Chamove & Anderson 1979; Bloornstrand et al 1986).
Methods
Subjects and housing
The subjects were a group of 17 juvenile female rhesus macaques (Macaca mulatta), housed in a large indoor/outdoor cage system. All observations took place in the outdoor cage (7x3x3m). Food (commercial primate pellets) and water were available ad libitum in the indoor area, and fresh fruit and vegetables were provided once or twice weekly.
Prior to the study, the subjects were assigned to one of three dominance subclasses (dominants, n = 5; intermediates, n = 7; subordinates, n = 5) on the basis of over 1,000 aggressive interactions used to calculate dominance indices, according to the procedure described by Zumpe and Michael (1986).
The 'swimming pool' was an 8mm. thick glass aquarium (l20x50x60cm high) protected along the edges and at the comers by an iron frame. For each test the aquarium was filled with clean water (23-27ºC at the start of the test) to a depth of 50cm.
Procedure
Sixteen days of testing were conducted over a three week period in September 1991, when daytime temperatures were judged to be sufficiently high to encourage swimming. 'Control' days (n = 8) consisted of days in which the swimming pool was not presented to the group. 'Pool' days (n = 8) were those on which the pool was available. 'Me eight pool days comprised four conditions. Due to weather constraints (i.e. the approaching end of warm summer days), each pool condition was presented only twice. The four conditions were chosen to give preliminary information regarding the possible effects of visibility of the submerged food (bananas) and of the presence of food that could only be harvested while swimming along the bottom of the pool (raisins). In the 'box' condition, at the start of the test an opaque PVC box containing eight pieces of banana was fixed to the bottom of the pool. The box could be opened by pivoting the lid horizontally. Two members of the group had previously learned to dive into the pool and open the box under water, thereby releasing the bananas (Anderson et al 1992). In the 'tubes' condition, instead of the box, two rows of four PVC tubes (diameter 2.5cm, height 5.5cm) were fixed vertically to the bottom of the pool, and one third of an unpeeled banana was put into each tube. Thus, unlike in the box condition, in the tubes condition the banana pieces were visible from the start of the test. In the 'raisins' condition, neither the box nor the tubes were present, but 50 raisins were spread along the floor of the pool before the test. Unlike in the pool tests with bananas, in the raisins condition food could only be obtained by collecting the raisins from the bottom of the pool; unlike bananas, the raisins did not float, and they could not easily be stolen from a subordinate by a more dominant subject after the former emerged from the pool. In the 9 no-food' condition, the pool contained only water. The order of testing was the same as that used to describe the conditions, with one control day following every pool day
Each day's testing consisted of four one hour tests; two in the morning and two in the afternoon. Before every pool test the subjects were confined to the indoor cage section while the pool was prepared, which took about 20 minutes, then released immediately before behavioural sampling. On control days the subjects were also confined indoors for about 20 minutes before each test.
During all tests, one or both observers scored social and individual behaviours including aggression (given and received), grooming, contact, play and cage manipulation, focusing on one subject at a time for 10 minutes and using a one/zero sampling procedure (Martin & Bateson 1986) with 30s intervals signalled by a metronome. During pool days, the second observer recorded the identity of all subjects engaging in any of the following water-related behaviours: swimming on the surface of the water; immersed (three limbs in the water, one limb holding on to the edge of the pool); head under water, and diving (resulting in the subject being fully submerged and usually followed by underwater swimming).
Analysis
The data from each day's four one hour tests were pooled. Those behaviours yielding sufficient data were analysed using within- and between-subjects analyses of variance, using social status (dominant, intermediate, subordinate) as the between-subjects factor and different combinations of conditions (control, pool, no-food, box, tubes, raisins) as repeated measures. Alpha was set at 0.05. All significant effects are reported below, as are some notable nonsignificant trends (0.10>P>0.05).
Results
Social and individual behaviour
There was a highly significant decrease in overall grooming activity on days when the pool was available, from an average of 26% of observation intervals to 10.6% (df = 1,14; F = 19.3; P<0.001). Decreases were also significant when the subjects were considered separately as givers and receivers of grooming (df = 1,14; F = 11.4 and 12.3; P<0.005 respectively). There were no significant effects involving dominance status, although the dominant
Subgroup consistently had the highest average grooming scores, and the subordinates the lowest. However, grooming activity was not affected differentially by the four pool conditions. Neither self-directed grooming nor passive social contact was influenced by any of the independent variables.
There were several significant effects in the analyses of agonistic behaviour. The dominant subgroup was the most likely to show aggression, followed by the intermediates and then the subordinates (df = 2,14; F = 6.4; P = 0.01); the inverse order held for receipt of aggression (df = 2,14; F = 5.5; P<0.025). Overall levels of aggression were low, with a non-significant increase in the presence of the pool (P = 0.67). This trend led us to analyse total aggression scores in the four different pool conditions. The only significant effect was due to condition, with mean aggression scores decreasing in the following order of conditions: tubes (0.8% of intervals), box (0.7%), raisins (0.4%), no-food (0.2%) W = 3,42; F = 3.2; P<0.05).
Social avoidance responses were four times more frequent in the subordinate subgroup than in the dominant subgroup, with avoidance scores of intermediate ranking subjects falling in between these two extremes (df = 2,14; F = 6.5; P=0.01). Occurrences of one subject avoiding the approach of another increased slightly but significantly in the presence of the pool (df = 1,14; F = 5.1;P<0.05); there was no interaction with subgroup.
The frequency of social play did not vary according to the presence or absence of the pool. Intermediate and subordinate ranking subjects played over twice as frequently as dominants (P =0.085). In contrast to social play, solitary (i.e. self-motion) play showed a tenfold increase in the presence of the pool (df = 1,14; F = 7.2; P<0.05), reaching an overall average of 1.8 per cent of observation intervals; there was no interaction involving dominance status. 'Me two categories of play were analysed across the different pool conditions; in both cases most play occurred in the tubes condition, while least play occurred in the box condition (social play: df = 3,42; F = 3.5; P<0.05; solitary play: df = 3,42; F = 3.6; P<0.05), with no other significant effects. Interestingly, manipulation of the cage structures decreased in the presence of the pool (df = 1,14; F = 7.2; P<0.025), with no effect of social status.
Water-related activities
The analysis of all water-related activities combined yielded an interaction between social status and condition which approached significance (P = 0.061). The corresponding means are shown in Figure 1. Several aspects of the data are noteworthy. The intermediate ranking subgroup engaged in most of the pool-related activities, especially in the raisins and no-food conditions. Subjects in the subordinate subgroup showed relatively little water-related activity in the three conditions in which food was present, but approximately doubled their water-related scores in the no-food condition. In contrast, dominant subjects showed almost no interest in the water when it contained no food. The presence of food, especially raisins, increased the dominants' overall interest in the pool.
The behaviour 'head under water' yielded no significant effects, either when all instances were analysed together or when different contexts of the behaviour - i.e. instances associated with obtaining or trying to obtain food, or non-food-related instances - were analysed separately. Simple immersion in the water and swimming on the surface were generally rare, and did not vary according to social status or conditions.
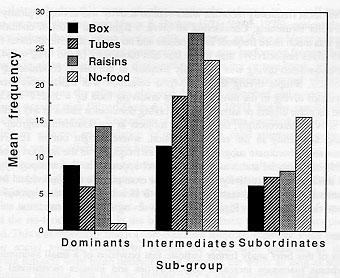

By far the most frequently recorded water-related activity was diving, typically associated with underwater swimming. Considering all dives, a significant effect of condition showed that diving was much more frequent in the raisins and no-food tests (overall mean frequencies of 9 and 10 dives respectively) than in the box and tubes tests (3 and 4) (df = 3,42; F = 4.4; P<0.01). Food-related diving was especially frequent in the raisins condition (df = 2,28; F = 4.0; P<0.05). Simple diving in the no-food. condition was much more frequent than non-food-related diving in the three conditions involving food (df = 3,42; F = 5.5; P<0.01). There were no main effects or interactions involving dominance status in any of the analyses of diving activity. Interestingly, however, all subjects in the dominant subgroup who dived did so most frequently in the raisins condition, whereas eight out of 10 divers in the intermediate and subordinate subgroups dived more frequently in the no-food condition. Any potential effects of social status at the group level appeared to be swamped by substantial individual differences in proclivity for diving. For example, the total individual frequency of diving, summed over all pool tests, ranged from 0 (3 subjects, 1 in each group) to 170 (an intermediate ranking subject). Figure 2 illustrates diving in the box condition.
Discussion
The results of this brief study further indicate that provision of a small swimming pool for juvenile rhesus macaques increases play activities and induces recreational diving and underwater swimming, while reducing cage manipulation and the overall incidence of social grooming. As the monkeys were already highly familiar with the pool (see Anderson et al 1992), novelty can be discounted as an important cause of the behavioural changes observed, although more extensive observations in the different experimental conditions would be desirable. Nevertheless, the present results taken with previous results on the same group indicate that a swimming pool is a useful environmental enrichment feature for captive macaques. Further data should be collected on the enrichment value of a pool for other species and other age-sex classes
Aggression was very rare under the conditions of the present study, but its frequency of occurrence was affected by different presentations of food incentives in the water. Overall water-related activities, especially diving and underwater swimming, were also affected by the food presented along with the pool. Both social rank (dominance subgroup membership) and especially individual differences, influenced use of the pool. These points are discussed below.
Reductions in social grooming have been reported to result from other environmental modifications aimed at increasing overall activity (Byrne & Suomi 1991; Parks & Novak 1993). Unlike social grooming, the frequency of social play was not affected by the presence of the swimming pool; nor was play affected by the provision of supplementary foraging opportunities in rhesus macaques (Byrne & Suomi 1991). In the pool condition of the present study, some bouts of social play occurred in relation to the water; for example, one monkey would leap out of the water on to the ground beside the pool and immediately be contacted playfully by another. Solitary play activities, which increased by a factor of 10 in the presence of the pool, were also frequently water-related. For example, a subject would leap out of the water and grab on to a swing or an elevated horizontal tree-trunk and engage in acrobatics. Interestingly, this type of play, variously referred to as self-motion play, peragration, or peregrination (see Mears & Harlow 1975; Smith 1978), noticeably increased in frequency before the first direct contact with the water, as if the subjects were somehow preparing themselves for subsequent water-play. It was also performed frequently by two or more subjects in close proximity, i.e. sharing the same swing or tree-trunk, giving it social overtones. Increased playfulness in response to the presence of novel objects, but not necessarily incorporating the stimulus object(s), has been described and commented upon in other animals, notably pigs (Wood-Gush & Vestergaard 1991), but the precise significance of the increase remains unclear. Regardless of the mechanisms involved, the increase in play accompanied by reduced manipulation of the familiar cage structures implies that the swimming pool was an effective enrichment apparatus.
Although agonistic behaviour was infrequent, it was more often observed when highly prized food items - pieces of banana - were present in the water, especially if they were clearly visible (tubes tests). Aggression was even less frequent when no food was present or when raisins were the food on offer. Interestingly, diving was also more frequent in the raisins and the no-food tests than in the box and tubes tests, in which banana pieces could be obtained. This was especially true for non-dominant subjects. Together, these results reflect the greater social tension created by baiting the pool with bananas. In these tests, lower-ranking subjects were more hesitant about entering the water as long as some banana pieces remained uneaten, lest they be punished by a more dominant subject. In fact, the alpha-ranking female of the group never entered the water, but obtained a disproportionate amount of the banana pieces by waiting for them to float to the surface after they had been disturbed by another individual (see Anderson et al 1992); her monopolization of the released food was sometimes backed up by threats directed towards others who came too near. In this context, it is noteworthy that the raisins condition did not appear to create much tension. This may be because raisins are less highly prized than bananas, but it may also be related to the fact that diving subjects often picked up the raisins and transferred them to their mouth while still swimming under water; thus the dominants could not chase them and force them to abandon the food when they emerged from the water, a scenario which did occur often with the bananas. These results are preliminary, but they suggest that visibility of submerged food and the ease with which a food item might be stolen can influence diving activities in a group of monkeys.
There were few effects of dominance subgroup membership on water-related activities, but, as noted above, lower ranking subjects tended to engage in more diving when there were no food incentives in the water. Overall, individual differences in taking to the water appeared more influential than dominance-related effects. Certain individuals never entered the water during the present study, while others literally took the plunge in almost every test. Such variability confirms the view that attempted environmental enrichment procedures should not reasonably be expected to produce similar behavioural changes in all individuals, or in all groups (Bloomstrand et al 1986; Anderson & Visalberghi 1990; Suomi & Novak 1991; Parks & Novak 1993). In particular, incorporating food into a spatially restricted enrichment apparatus may increase the potential for conflict, and compromise access for certain subjects.
Animal welfare implications
When a small swimming pool was available, the members of a group of young rhesus monkeys engaged in less social grooming and cage manipulation, and more play activities. Most researchers would agree that such behavioural changes are positive for captive macaques. Most of the monkeys engaged in diving and recreational underwater swimming. Of the different food conditions used, spreading raisins on the bottom of the pool appeared the most successful for eliciting diving without increasing aggression. Individual differences, food presentation, and possibly social status interacted to influence access to and use of the swimming pool, which appears to be an excellent form of environmental enrichment for captive rhesus macaques.
Acknowledgements
This research was carried out at the Centre de Primatologie, Université Louis Pasteur, Strasbourg. We thank personnel of the Centre for their help, and M Anthouard for providing and maintaining the aquarium.
References
Anderson J R, Peignot P and Adelbrecht C 1992 Task-directed and recreational underwater swimming in captive rhesus monkeys (Macaca mulatta). Laboratory Primate Newsletter 31 (4): 14
Anderson J R and Visalberghi E 1990 Towards better conditions for captive nonhuman primates: routines, requirements, and research. In Alleva E and Laviola G (eds) Biomedical Experimentation and Laboratory Animals: Hot Behavioural Issues pp 1-11. ISTISAN Reports: Rome
Baldwin J D 1986 Behavior in infancy: exploration and play. In Mitchell G and Erwin J (eds) Comparative Primate Biology Vol 2A: Behavior, Conservation, and Ecology pp 295-326. Alan R Liss: New York
Berman C 1977 Seaside play is a serious business. New Scientist 73: 761-763
Bloomstrand M, Riddle K, Alford P and Maple T L 1986 Objective evaluation of a behavioral enrichment device for captive chimpanzees (Pan troglodytes). Zoo Biology 5: 293-300
Byrne G D and Suomi S J 1991 Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. American Journal of Primatology 23: 141- 15
Chamove A S and Anderson J R 1979 Woodchip litter in macaque groups. Journal of the Institute of Animal Technicians 30: 69-7
Fittinghoff N A and Lindburg D G 1980 Riverine foraging in east Bornean Macaca fascicularis. In Lindburg D G (ed) The Macaques pp 182-214. Van Nostrand Reinhold: New York
Gautier-Hion A 1973 Social and ecological features of talapoin monkey - comparisons with sympatric cercopithecines. In Michael R P and Crook J H (eds) Comparative Ecology and Behaviour of Primates pp 147-170. Academic Press: New York
Gilbert S G and Wrenshall E 1989 Environmental enrichment for monkeys used in behavioral toxicology studies. In Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 244-254. Noyes Publications: Park Ridge, NJ
Hamilton W J 111 1982 Baboon sleeping site preferences and relationships to primate grouping patterns. American Journal of Primatology 3: 41-53
Kawai M 1965 Newly-acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima Islet. Primates 6: 1-3
Lindburg D G 1971 The rhesus monkey in north India: an ecological and behavioral study. In Rosenblum L A (ed) Primate Behavior Vol I pp 1-106. Academic Press: New York
Martin P and Bateson P 1986 Measuring Behaviour. Cambridge University Press: Cambridge
Mears C E and Harlow H F 1975 Play: early and eternal. Proceedings of the National Academy of Sciences, USA 72. 1878-1882
Parks K A and Novak M A 1993 Observations of increased activity and tool use in captive rhesus monkeys exposed to troughs of water. American Journal of Primatology 29: 13-25
Poole T B 1988 Normal and abnormal behaviour in captive primates. Primate Report 22: 3-12
Roonwal M L and Mohnot S M 1977 Primates of South Asia. Harvard University Press: Cambridge, MA
Smith E 0 (ed) 1978 Social Play in Primates. Academic Press: New York
Suomi S and Novak M A 1991 The role of individual differences in promoting psychological well- being in rhesus monkeys. In Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 50-56. American Psychological Association: Washington, D C
Wood-Gush D G M and Vestergaard K 1991 The seeking of novelty and its relation to play. Animal Behaviour 42: 599-606
Yeager C P 1991 Possible antipredator behavior associated with river crossings by proboscis monkeys (Nasalis larvatus). American Journal of Primatology 24: 61-66
Zumpe D and Michael R P 1986 Dominance index: a simple measure of relative dominance status in primates. American Journal of Primatology 10: 291-300
This article originally appeared in Animal Welfare 3: 275-283 (1994).
Reprinted with permission of the publisher.