S J Schapiro, S A Suarez, L M Porter and M A Bloomsmith
Department of Veterinary Sciences, University of Texas MD Anderson Cancer Center
Science Park, Bastrop, Texas 78602 USA
Abstract
Captive primates rarely have to spend as much time searching for, obtaining and processing food as do their wild counterparts. Enrichment techniques designed to encourage captive primates to spend more species-appropriate amounts of time in foraging behaviours have been successful. The present study measured the behavioural effects of four feeding enhancements: two devices (mats and puzzles) and two foods (produce and frozen juice), on four cohorts (n = 63) of single-caged, yearling rhesus macaques (Macaca mulatta). Devices required considerable manipulation to retrieve rewards, whereas enrichment foods required additional processing. Analyses compared periods when one of the enhancements was available to interim periods when no enhancements were available. Planned comparisons revealed that subjects spent more time feeding, and less time inactive, self-grooming, exploring and behaving socially when feeding enrichment was available. Significantly more time was spent feeding when enrichment foods were provided, but more time was spent playing and using enrichment when devices were in the cage. More time was spent self-grooming and exploring with the acrylic puzzle than with the artificial turf mat. Subjects spent significantly more time feeding when produce was available than when frozen juice was available. Feeding enhancements resulted in more species-typical patterns of activity for single-caged, yearling rhesus. Since feeding devices were used in species-typical activities in addition to feeding, devices may be more valuable than foods. Feeding enrichment programmes which combine stimulating devices with foods that are novel and require processing can positively affect the behaviour of captive primates.
Keywords: animal welfare, feeding, feeding enrichment, Macaca mulatta, psychological well-being, single caging
Introduction
Primates living in the wild spend as much as 65 per cent of their time searching for, obtaining, processing and eating food (Clutton-Brock & Harvey 1977; Wrangharn 1977; Milton 1980;Altmann & Muruthi 1988; Malik & Southwick 1988; Watts 1988). Captive primates, particularly those given a pelleted diet, spend considerably less time engaged in activities related to feeding (Bloomsmith et al 1988; Reinhardt 1993, 1994; Schapiro & Bloornsmith 1995). Even though these animals receive a balanced and nutritious diet, they may be deprived of one of their most important species-typical and time-consuming occupations: 'working' for their nourishment.
One of the more popular, well-studied and effective techniques for enriching the environments of captive primates is to provide them with opportunities to increase the amount of time they can spend engaged in naturalistic foraging behaviours (Beckley & Novak 1989; Bloomsmith 1989; Brent & Eichberg 1991; Reinhardt 1993; Bayne et al 1994; Lambeth & Bloomsmith 1994). This can be accomplished by increasing the number (and decreasing the size) of meals (Bloomsmith et al 1988), moving the food dish (Reinhardt 1994), providing novel foods (Schapiro & Bloomsmith 1995), or designing simple or complex, species-relevant problems to obtain food (Nash 1982; Bloomsmith et al 1988; Visalberghi & Vitale 1990; Bayne et al 1992). Many of these techniques result in higher levels of species-typical behaviours including time spent feeding (Chamove et al 1982; Hayes 1990; Byrne & Suomi 1991; Schapiro & Bloomsmith 1994, 1995), and lower levels of abnormal behaviours (Bloomsmith et al 1988; Bayne et al 1991; Brent & Eichberg 1991). These results lead to the conclusion that various feeding enhancements can improve the well-being of captive primates (Bayne 1991; Lindburg 1991). When designing feeding enrichment programmes, potential occupational benefits are usually emphasized more than nutritional benefits (Bloomsmith et al 1988; Beckley & Novak 1989; Hayes 1990; Byrne & Suomi 1991).
The work of Andrews, Rosenblum and their colleagues (Plimpton et al 1981; Andrews & Rosenblum 1988, 1991) provides a theoretical context in which to place applied studies of the effects of feeding enrichment on behaviour patterns indicative of well- being. They have clearly demonstrated that the difficulty in finding food (foraging demand) influences many aspects of the behaviour, social relationships and social development of captive primates. In general, their studies have shown that high or variable foraging demands lead to a variety of negative behavioural outcomes, suggesting that feeding enhancements which lower foraging demand might be expected to result in few negative outcomes and might even yield some positive behavioural effects.
Previously, we have compared the behaviour of single-caged, yearling rhesus that did not receive any enrichment to the behaviour of subjects that had received a three-phase (physical, feeding and sensory) enrichment programme (Schapiro & Bloomsmith 1995; Schapiro et al 1995). In addition to diminished self-aggression and increased play, the feeding enrichment programme resulted in the highest mean durations spent feeding (Schapiro & Bloomsmith 1995). The present study extends these analyses by more closely examining the behavioural effects of four individual feeding enhancements.
Methods
Subjects and housing
Yearling rhesus macaques from cohorts born in 1988, 1989, 1990 and 1991 (groups 1-4, Schapiro & Bloomsmith 1995; Schapiro et al 1995) were observed while single housed. All monkeys (n = 63) had been socially housed during their first year of life (Schapiro et al 1994). All the monkeys were provided with biscuits twice daily, and supplemental pieces of orange were given three times per week. Water was available ad libitum. Subjects were housed in rack-mounted stainless steel cages (0.4m2) with visual, auditory and olfactory access to similarly housed monkeys. For each cohort, the enriched subjects received an environmental enrichment programme that consisted of three separate enrichment conditions during the year of single housing.
Enrichment
Monkeys sequentially received three different types of enrichment. For each of the four cohorts included in this study, feeding enrichment (devices and foods) lasted six months and occurred at either the beginning, in the middle or at the end of the year of single caging. (For additional details on the three-phase enrichment programme, see Schapiro & Bloomsmith 1995.) The feeding enrichment programme was designed to add variety to the monkeys' standard pelleted diet, to encourage the animals to express their natural food discovery and processing skills, and to increase the amount of time that they spent in foraging activities. Thus, we presented five feeding enhancements (three feeding devices and two enrichment foods) to each monkey every weekday for the six months of this enrichment phase. A feeding device was defined by the fact that subjects had to search for food in or on it prior to being able to eat the food. Enrichment foods were simply placed inside the cage. The two enrichment foods used were fresh produce and frozen juice. A daily ration of produce weighed about 125g and was comprised of approximately 40 per cent vegetable and 60 per cent fruit. Produce items were rotated weekly, and during the course of this study, monkeys received over 40 different fruits and vegetables, including carrots, potatoes, cantaloupe and watermelon. Frozen juice was presented as a 100ml block of sugared drink mix; eight different flavours were used. The two feeding devices studied were an artificial turf foraging mat and a clear acrylic foraging puzzle (Schapiro et al 1991; Schapiro & Bloomsmith 1995). Mats and puzzles were loaded with 20g of seeds or grain (sunflower seeds, peanuts, popcorn, hen scratch or whole corn) each time they were presented. Subjects had to pick seeds from within the 'blades' of the artificial turf and shake seeds through a series of internal holes until they reached the opening of the puzzle. Foods retrieved from feeding devices frequently required additional processing by the subjects (corn had to be cracked and sunflower seeds had to be hulled etc.).
Enrichment items were given to the monkeys at 1.5 hour intervals commencing at 0830h and ending at approximately 1600h. The order of presentation of the enhancements was rotated regularly. When one item was put in the cage, the previous item (if a device) was removed. Enrichment foods rarely remained in the cage long enough to be removed at the beginning of the next interval.
Data collection
Fifteen-minute focal animal observations (Altmann 1974) were conducted on all subjects, using a Tandy© 102 portable computer and The Observer© (Noldus 1991), an observational software package. Subjects were observed approximately twice per week, resulting in 710 hours of data from the four cohorts during the feeding enrichment phase. Data were collected between 0830h and 1730h throughout the year, were balanced for time of day, and included periods when feeding enrichment was available and when it was not (Schapiro & Bloomsmith 1995).
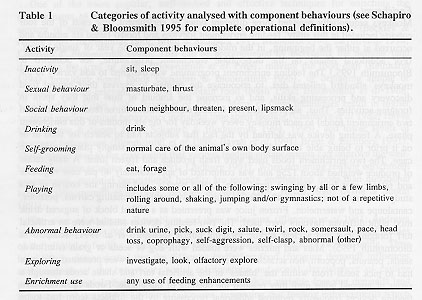
Data were recorded as 36 individual behaviours according to our standard ethogram (see Table 1 and Schapiro & Bloomsmith 1995) and were grouped into nine categories of activity for analysis. Enrichment use was recorded simultaneously with all other focal animal behaviour. Inter-observer reliability for cumulative durations of behaviours within observation sessions was measured monthly for a total of seven observers and averaged 88.3 per cent for behaviour and 95.1 per cent for enrichment use.
Data analysis
There were four feeding enhancements studied, plus a 'control' condition for comparison. The control condition was comprised of observations conducted during interim periods, outside the regular 1.5 hour intervals during which a feeding enhancement was presented. These interim periods included: 1) early morning observations before the first enhancement, 2) observations just prior to the early afternoon cleaning procedures, 3) late afternoon observations after the last enhancement period, and 4) other periods when, for a specific reason, no feeding enrichment was given to the monkeys.
A repeated-measures multivariate analysis of variance (MANOVA) was performed with five levels of the within-subjects factor: 1) the interim period, 2) acrylic puzzle available, 3) turf mat available, 4) fresh produce available, and 5) frozen juice available. The dependent measures analysed were the mean cumulative durations of the nine target activities (see Table 1) per subject for each of the five feeding enhancement conditions. Several sets of planned comparisons were established prior to analysis: the comparison of the interim period to the four feeding enhancements combined; the comparison of the two feeding devices to the two enrichment foods; the comparison of the puzzle to the mat; and the comparison of produce to frozen juice. In addition, a repeated-measures analysis of variance (ANOVA) was performed with enrichment use as the dependent measure, utilizing the same five levels of the within-subjects factor and the same planned comparisons.
Results
Overall, there were differences in behaviour across the five feeding conditions (F(44,2728) = 168.5, P £ 0.001). Univariate tests revealed that inactivity (F(4,248) = 12.2, P £ 0.001), social behaviour (F(4,248) = 5.3, P £ 0. 00 1), self-grooming (F(4,248) = 12. 1, P £ 0.001), feeding (F(4,248) = 29.8, P £ 0.001), playing (F(4,248) = 29.6, P £ 0.001) were all significantly affected. These findings were neither unexpected nor particularly interesting, but were important because they permitted us to conduct the more theoretically meaningful planned comparisons.
The comparison of the interim condition to the statistical combination of the four feeding enhancements (see Table 2) indicated that the devices and foods resulted in decreased time spent: inactive (F(1,62) = 26.9, P £ 0.001), engaged in social behaviour (F(l, 62) = 16.8, P £ 0.001), self-grooming (F(1,62) = 30.9, P £ 0.001), and exploring (F(1,62) = 91.4, P £ 0.001). Time spent feeding was significantly greater when enhancements were available than when they were not (F(1,62) = 90.9, P £ 0.001). Abnormal behaviour was not affected (F(l, 62) = 1. 4, P > 0. 05).
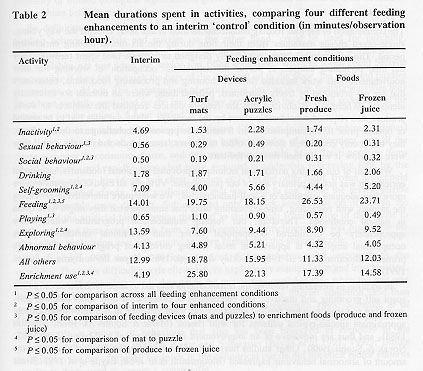
The comparison of the two feeding devices to the two enrichment foods revealed that the presence of the devices resulted in increased time spent in sexual behaviour (F(1,62) = 9.8, P £ 0.01) and play (F(1,62) = 28.7, P £ 0.001), and decreased time spent in social (F(1,6 = 4.5, P £ 0.05) and feeding behaviour (F(l, 62) = 34.0, P £ 0.00 1).
Subjects spent more time self-grooming (F(1,62) = 16.3, P £ 0.001) and exploring (F(1,62) = 13.8, P £ 0.001) when the acrylic puzzles were in the cage than when the artificial turf mats were accessible.
Significantly more time was spent feeding (F(1,62) = 3.9, P £ 0.05) during intervals when produce was available than when frozen juice was provided.
Enrichment use differed significantly across the five enhancement conditions (F(4,248) = 69.4, P £ 0.001). Significantly more time was spent using enrichment when the four feeding enhancements were available than during the interim condition (F(1,62) = 295.2 P £ 0.001), and devices were used more than food (F(1,62) = 41.3, P £ 0.001), with the subjects using the mat the most (F(1,62) = 10.5, P £ 0.01). Time spent using produce and frozen juice did not differ 01,62) = 3.6, P > 0.05).
Discussion
The combination of the four feeding enhancements used in this study affected the way young, single-caged rhesus macaques spent their time during the six-month feeding enrichment period. The enhancements were specifically designed to increase time spent feeding. They did this, permitting subjects to pursue the naturalistic 'occupation' of working for their nourishment. This work included finding, choosing and processing food items, behaviours that are not promoted by freely - distributed, pelleted diets, where all biscuits are essentially identical. Whereas it is obvious that the feeding devices required the subjects to work, produce also required considerable effort by the monkeys. Many varieties had to be peeled or opened prior to consumption. Even frozen juice presented a challenge to the monkeys: they could only consume it slowly in order to prevent frozen hands and mouths, but if they were too slow it would melt through the bars.
While all of our feeding enrichment techniques provided additional foodstuffs, nutritional enrichment was not the primary aim of our programme. Virtually all captive primates receive more than adequate quantities of a well-balanced diet. We were more interested in providing the animals with rewarding, time-consuming tasks that simulate what they might have to do in natural conditions. Therefore, our feeding enhancement programme would more appropriately be considered occupational rather than nutritional enrichment. This occupational emphasis is apparent in most feeding enrichment programmes for captive primates (Bloomsmith et al 1988; Beckley & Novak 1989; Hayes 1990; Byrne & Suomi 1991).
In addition to increasing feeding, the enhancements also led to important decreases in time spent self-grooming and inactive. The percentages of observation time spent feeding, self-grooming and inactive when feeding enhancements were available in this study more closely approximate species-typical patterns for wild rhesus (Malik & Southwick 1988; Marriott 1988), and thus are indicative of an improvement in psychological well-being (Line 1987; Novak & Suomi 1988). Other studies have reported that feeding enrichment decreased the amount of abnormal behaviour expressed (Bloomsmith et al 1988; Bayne et al 1991; Brent & Eichberg 1991), but neither the current analyses, nor any of our other analyses (Schapiro & Bloomsmith 1994, 1995; Schapiro et al 1995) have detected an effect of inanimate enrichment on abnormal behaviour.
Expectedly, time spent using enrichment increased when one of the devices or foods was available. Subjects could use enrichment, even during the interim periods when no feeding enhancements were available in the cage, because enrichment foods (produce and seeds from enrichment devices) that were dropped out of the cage often fell in the pan beneath. Monkeys could be recorded as foraging for enrichment remnants in the pan at any time.
Subjects spent significantly more time playing and using enrichment when devices were present than when foods were, but spent more time feeding when foods were available. The presentation of enrichment foods led to large increases in the amount of time spent feeding, an important enhancement for captive primates. But increased time spent playing and using enrichment, suggest that feeding devices that require the monkeys to work, may lead to better approximations of multiple components of the species-typical behavioural repertoire. The observed changes in sexual and social behaviour, although statistically significant, are probably of limited biological significance, given the small mean amount of time accounted for by these behaviours.
By definition, the only behaviours that subjects could direct at enrichment foods were feeding behaviours. Mats and puzzles on the other hand, could be used for perching, manipulating, exploring and playing. These activities could occur at any time while a device was available, not just when the device was filled with food. Whereas foods could become inaccessible if they fell through the bars of the cage and on to the floor, the mats and puzzles themselves, could not. The increased use of enrichment devices in non- feeding activities may be at least partially explained by the fact that they remained in the cage and accessible to the monkeys for the entire 1.5-hour period.
We were next interested in seeing if there were differences in the way subjects behaved when each device or food item was present. In both categories of enhancement, one of the items was easy to consume or use, and one was more difficult. Many fresh produce items could be easily and rapidly eaten, but some required considerable processing time by the subjects (e.g. celery, kiwi fruit, bok choy, lemons etc.). On the other hand, frozen juice could only be consumed slowly. The artificial turf mat was easy to use, while the acrylic puzzle was considerably more difficult. Subjects had only to pick seeds from on or within the 'blades' of the artificial turf, whereas they had to shake seeds from the top of the puzzle to the bottom and then extract them from a small opening. Enrichment use was higher for the mat, while exploration and self-grooming were higher for the shaker puzzle.
Successful use of the two devices required different behavioural responses by the monkeys. The more difficult puzzle elicited more exploratory manipulation and investigation. Time spent self-grooming was higher when the puzzle was available than when the mat was in the cage, but was still significantly lower than during the interim condition (and than among control (never enriched) subjects (Schapiro & Bloomsmith 1995; Schapiro et al 1995). Monkeys were often observed picking through an empty mat, as if they were grooming it, a pattern of behaviour that also has been observed in response to the grooming/foraging boards used by Bayne and colleagues (Bayne et al 1991, 1992). Unlike foraging on the mat foraging for seeds in the cylindrical puzzle did not elicit motor patterns similar to those used to groom a conspecific.
Andrews, Rosenblum and their colleagues (Plimpton et al 1981; Andrews & Rosenblum 1988, 1991, 1993) have demonstrated in captive macaques how the behaviour, development and ability to respond to challenges can be influenced by how difficult it is to procure food. In their studies, conditions of high or variable foraging demand (harder to find food) led to more negative behavioural outcomes than did low foraging demand conditions (easy to find food, Andrews & Rosenblum 1988, 1991). In these studies, variations in how difficult it was to obtain the pelleted diet determined the level of foraging demand. The devices that we used required the macaques to work to obtain highly desirable food, but this food was always presented in addition to their generous ration of pelleted diet. Providing enrichment foods and allowing subjects to work at the mildly challenging devices, in addition to having free access to pellets, may have created what might be considered a 'very low and very exciting foraging demand' condition. This condition, which promoted species-typical behaviours to obtain the desirable foods when an enhancement was present, may account for the positive behavioural outcomes that were observed. Previous analyses of the effects of the overall feeding enrichment programme support this suggestion, and indicate an impact of feeding enrichment procedures that extend beyond periods when enhancements are available (Schapiro & Bloomsmith 1995; Schapiro et al 1995). Foraging demand (Andrews and Rosenblum 1988, 19919 1993) may provide an appropriate context within which the results from applied investigations of feeding enrichment can be interpreted.
We feel that a feeding enrichment programme similar to the one that we used, that provides some combination of stimulating devices and foods that are novel and require processing, can have a very positive impact on the behaviour of captive primates. We have used a similar feeding enrichment programme for older, pair-housed and group-housed rhesus with less success (Schapiro et al 1993; Schapiro & Bloomsmith 1994), suggesting that young, single-caged subjects may benefit most from this type of enrichment regimen.
Animal welfare implications
Feeding devices and enrichment foods were responded to differently by young, single- caged rhesus macaques. Devices served multiple enrichment functions for socially- restricted monkeys: empty mats and puzzles became perches and toys, providing opportunities to express more components of the species-typical behavioural repertoire than did a piece of banana or a block of frozen juice. Similarly, the different devices elicited different behavioural responses depending on how difficult and how portable they were. Thus, if an increase in the amount of time that monkeys spend feeding is the goal of an enrichment programme, then the simple presentation of enrichment foods would be appropriate. But in instances where an increase in time spent in other species- typical behaviours is desired as well, presenting monkeys with devices that can be used in other activities and that contain foods that require some additional processing may be particularly valuable.
Acknowledgements
We thank A Kessel, M McDonald, and D Oubari for dedicated collection of data and D Glasgow and C J Malinierni for data management efforts. We also thank J McMahan, S Buchl and the rest of the primate section staff. Animals are maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health regulations and standards. Financial support for this project came from the National Institutes of Health/National C t f Resources grants U42-RRO5080 and R01-RR05092.
References
Altmann J 1974 Observational study of behavior: sampling methods. Behaviour 49: 227- 267
Altmann J and Muruthi P 1988 Differences in daily life between semiprovisioned and wild-feeding baboons. American Journal of Primatology 15:.213-221
Andrews M W and Rosenblum L A 1988 Relationship between foraging and affiliative social referencing in primates In: Fa J E and Southwick C H (eds) Ecology and Behavior of Food-Enhanced Primate Groups pp 247-268. Alan R Liss: New York, USA
Andrews M W and Rosenblum L A 1991 Security of attachment in infants raised in variable- or low-demand environments. Child Development 62: 686-693
Andrews M W and Rosenblum L A 1993 Assessment of attachment in differentially reared infant monkeys (Macaca radiata): response to separation and a novel environment. Journal of Comparative Psychology 107: 84-90
Bayne K 1991 Alternatives to continuous social housing. Laboratory Animal Science 41: 355-359
Bayne KAL, Strange GM and Dexter SL 1994 Influence of food enrichment on cage side preference. Laboratory Animal Science 44: 624-629
Bayne K, Dexter S, Mainzer H, McCully C, Campbell G and Yamada F 1992 The use of artificial turf as a foraging substrate for individually housed rhesus monkeys (Macaca mulatta). Animal Welfare 1: 3953
Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F and Suomi S 1991 The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology 23: 23-35
Beckley S and Novak M 1989 Examination of various foraging components and their suitability as enrichment tools for captively housed primates. American Journal of Primatology Supplement 1: 37-43
Bloomsmith M A 1989 Feeding enrichment for captive great apes. In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 336-356. Noyes Publications: Park Ridge, USA
Bloomsmith M A, Alford P L and Maple T L 1988 Successful feeding enrichment for captive chimpanzees. American Journal of Primatology 16: 155-164
Brent L and Eichberg J W 1991 Primate puzzleboard: a simple environmental enrichment device for captive chimpanzees. Zoo Biology 10: 353-360
Byrne G D and Suomi S J 1991 Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus monkeys. American Journal of Primatology 23: 141-151
Chamove A S, Anderson J R, Morgan-Jones S C and Jones S P 1982 Deep woodchip litter: hygiene, feeding, and behavioral enhancement in eight primate species. International Journal of Studies on Animal Problems 3: 308-318
Clutton-Brock T H and Harvey P H 1977 Species differences in feeding and ranging behaviour in primates. In: Clutton-Brock T H (ed) Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes pp 557-584. Academic Press: London, UK
Hayes S L 1990 Increasing foraging opportunities for a group of captive capuchin monkeys (Cebus capucinus). Laboratory Animal Science 40: 515-519
Lambeth S P and Bloomsmith M A 1994 A grass foraging device for captive chimpanzees (Pan troglodytes). Animal Welfare 3: 13-24
Lindburg D G 1991 Ecological requirements of macaques. Laboratory Animal Science 41: 315-322
Line S W 1987 Environmental enrichment for laboratory primates. Journal of the American Veterinary Medical Association 190: 854-859
Malik I and Southwick C H 1988 Feeding behavior and activity patterns of rhesus monkeys (Macaca mulatta) at Tughlaqabad, India. In: Fa J E and Southwick C H (eds) Ecology and Behavior of Food Enhanced Primate Groups pp 95-111. Alan R Liss: New York, USA
Marriott B M 1988 Time budgets of rhesus monkeys (Macaca mulatta) in a forest habitat in Nepal and on Cayo Santiago. In: Fa J E and Southwick C H (eds) Ecology and Behavior of Food-Enhanced Primate Groups pp 125-149. Alan R Liss: New York, USA
Milton K 1980 The Foraging Strategies of Howler Monkeys: A Study in Primate Economics. Columbia University Press: New York, USA
Nash V J 1982 Tool use by captive chimpanzees at an artificial termite mound. Zoo Biology 1: 211-221
Noldus L P J J 1991 The observer: a software system for collection and analysis of observational data. Behavior Research Methods, Instruments, and Computers 23: 415- 429
Novak M A and Suomi S J 1988 Psychological well-being of primates in captivity. American Psychologist 43: 765-773
Plimpton E H, Swartz K B and Rosenblum L A 1981 The effects of foraging demand on social interactions in a laboratory group of bonnet macaques. International Journal of Primatology 2: 175-185
Reinhardt V 1993 Enticing nonhuman primates to forage for their standard biscuit ration. Zoo Biology 12: 307-312
Reinhardt V 1994 Caged rhesus macaques voluntarily work for ordinary food. Primates 35: 95-98
Schapiro S J and Bloomsmith M A 1994 Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. American Journal of Primatology 32: 159-170
Schapiro S J and Bloomsmith M A 1995 Behavioral effects of enrichment on singly- housed, yearling rhesus monkeys: an analysis including three enrichment conditions and a control group. American Journal of Primatology 35: 89-101
Schapiro S J, Bloomsmith M A, Porter L M and Suarez S A 1993 Housing conditions and/or age more strongly affect the behavior of young rhesus monkeys than does inanimate enrichment. American Journal of Primatology 30: 346
Schapiro S J, Brent L Y, Bloomsmith M A and Satterfield WC 1991 Enrichment devices for nonhuman primates. Lab Animal 20: 22-28
Schapiro S J, Lee-Parritz D E, Taylor L L, Watson L, Bloomsmith M A and Petto A 1994 Behavioral management of specific pathogen-free (SPF) rhesus macaques: group formation, reproduction, and parental competence. Laboratory Animal Science 44: 229-234
Schapiro S J, Porter L M, Suarez S A and Bloomsmith M A 1995 The behavior of single- caged, yearling rhesus monkeys is affected by the environment outside of the cage. Applied Animal Behaviour Science 45: 151-163
Visalberghi E and Vitale A F 1990 Coated nuts as an enrichment device to elicit tool use in tufted capuchins (Cebus apella). Zoo Biology 9: 65-71
Watts D P 1988 Environmental influences on mountain gorilla time budgets. American Journal of Primatology 15: 195-211
Wrangham R W 1977 Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In: Clutton-Brock T H (ed) Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes pp 503-538. Academic Press: London, UK
Printed with permission of the publisher.
This article originally appeared in Animal Welfare 1996, 5: 129-138