By Zeta Steen
School of Biological Sciences, Flinders University of South Australia
GPO Box 2100, Adelaide, SA, Australia
(Correspondence address: 9 Maidment Court, Wynn Vale, SA 5127, Australia.)
Introduction
Once an animal has been removed from its natural habitat every effort should be made not only to simulate the natural environment but also to ensure that the animal displays ecologically valid behaviour (Forthman Quick, 1984). Neither natural settings nor natural behaviour can be duplicated in captivity; however, complex captive settings may discourage the development of abnormal behaviour, induce activity and facilitate normal social behaviour and reproduction (Clarke et al., 1982). Markowitz (1975-79,1982, cited in Forthman Quick, 1984) has agreed that captive animals should exert some form of control over their environment. Control can be achieved by incorporating apparatus that requires animals to forage with increased effort to obtain food. The animals should also be able to have an element of choice in, for example, the ambient temperature and whether they are in sunshine or shade, as well as opportunities for movement, exploration and play (UFAW, 1990). The aim is to maximise the acquisition and practise of naturally occur- ring skills, enabling the animals to make use of as many social and environmental opportunities as are available. Such aims would help to prepare animals for participation in projects such as the Golden Lion Tamarin Reintroduction Programme (Redshaw and Mallinson, 1991). Enrichment devices can be used as effective means of increasing environmental complexity and allowing opportunity for expression of a greater range of primate behaviour in captivity (Bloomstrand et al., 1986). In this report we present the results of a project aimed to enrich food acquisition in the captive environment of two endangered species.
We examined two species of Callitrichidae, the cotton-top tamarin (Saguinus oedipus) and the golden lion tamarin (Leontopithecus r. rosalia). Both species are recognised as endangered. Population numbers of cotton-top tamarins in northern Colombia are at a critical level due to habitat destruction (Mittermeir, 1982, cited in Glatston et al.,1984). In Brazil, near Rio de Janeiro, cocoa and banana plantations, forestry, and housing developments are destroying tropical rain forests inhabited by golden lion tamarins (MacDonald, 1984); the animals originally inhabited primary forests, but because of degradation and 284 , destruction are being forced to live in poor secondary communities (Coimbra-Filho, in Kleiman, 1977). Coimbra-Filho captured golden lion tamarins in these areas and found them to be in poorer condition than captive born and raised animals, with smaller body size and inferior pelage in terms of colour and shine. Captive programmes may therefore be very important to the survival of these tamarin species, because they may provide stronger and 'fitter' animals than occur in existing natural habitats.
In the wild, many primates spend a large proportion of their day searching for food (Table 1). Golden lion tamarins are manipulative foragers. In comparison cotton-top tamarins exhibit highly opportunistic foraging patterns (Table 2 [not reproduced]). In the wild, cotton-top tamarins are most active in the densely foliated middle to lower canopy. They can live in semi-deciduous dry forests as well as tropical evergreen rain forests in primary or secondary growth areas (MacDonald, 1984). Both species have similar dentition and diet, but differ in size (Table 2 [not reproduced]). Differences observed in foraging time and technique may affect the way in which these two species respond to environmental enrichment. This could be important in reintroduction programmes because captive-born animals from the same family may need different forms of enrichment to promote 'natural' behaviour.
Differences in the physiological responsiveness of species to environ- mental change is known to influence the ways in which they respond to changes in their physical environment. In a comparative study of two similarly sized cebid species, the squirrel monkey (Saimiri sciureus) and the titi (Callicebus moloch), Box (1991) found that although the species eat similar foods and are often sympatric, they differ in their response to environmental challenges. Fragaszy and Adams-Curtis (1991) also noted differences in captive foraging techniques of squirrel monkeys and capuchin monkeys (Cebus apella). The squirrel monkey inspects surfaces by licking, sniffing and touching, maximising the probability of detecting small objects on newly encountered surfaces. Capuchins, however, are persistent manipulators and behave in ways that maximise the probability of discovering hidden or embedded objects.
One aspect of enhancing captive environments involves manipulation of food availability. Researchers have devised various mechanisms, constructions and situations that require an animal to 'work' for its food (McKenzie et at., 1986). For example, a suspended tube which drops mealworms at irregular intervals has been demonstrated to promote foraging on the ground in meerkats, promoting natural behaviour (UFAW, 1990). McKenzie et at. (1986) discovered that cotton-top tamarins made infrequent visits to the ground in a captive setting. By covering the bare cement floor with a deep layer of woodchips scattered with grain and insects, the number of visits to the ground was increased and became similar to that of wild counterparts. Another technique requiring animals to work for their food was used by Scott (1991). Aperspex bowl filled with sawdust and pieces of malt cake encouraged foraging. Some marmosets were reported to forage for up to six hours in this apparatus when housed individually (Scott, 1991).
The golden lion tamarin reintroduction programme attempts to provide a natural habitat and training for the animals (Redshaw and Mallinson, 1991). One training device used is a feeder constructed from lengths of plastic tubing. The tubing is filled with food and has holes along the side which allow foraging and access to the food. This type of technique allows food to be encountered in simulated nest holes so that: foraging becomes a habit in captive animals. It is often assumed that I 'fake' scenery (that appears natural) is best for enhancing the captive environment. An artificial substitute, however, such as a plastic feeder , may be a more effective substitute if it allows the animal to display natural behaviour in captivity (UFAW, 1990). Increased efforts in food acquisition lead to favourable behavioural consequences, such as reduced aggression and inactivity and are an efficient way of improving the quality of captive existence (McKenzie et at., 1986), Thus simple manipulations of the physical environment can alter the behaviour of captive animals, promoting behaviour that is more similar to that of wild counterparts (Fragaszy and Adams-Curtis, 1991).
The aim of this project was to investigate whether the introduction of enrichment apparatus would result in increased foraging effort by cotton-top tamarins and golden lion tamarins at Adelaide Zoo. Prior to the study the animals obtained food from a food bowl, rarely foraged, and spent a great deal of time resting or sitting and scanning the environment. As corollaries to the main aim, I investigated whether introduction of devices resulted in decreased scanning behaviour, whether the two species responded differently to the apparatus in terms of foraging and scanning time, and if an increase in types of devices induced further increases in foraging.
METHOD
Animals
All the animals used in this study were housed at Adelaide Zoo. The cotton-top tamarins were JJ (male, three years old), Parr (male, 15 years) and Hetti (female, 3 years 8 months). The golden lion tamarins were twin females (4 years 8 months old) respectively referred to as 'Dye' and 'Nodye'. Individuals were identified by the presence or absence of purple dye on limbs or tail. All the animals were captive-born and raised by their parents.
Behavioural classifications
The behaviours used in this study are defined as follows:
Foraging: There were different types of foraging. Natural foraging involved manipulation of leaves (plastic, silk or real), bark or wood with teeth or hands, as well as pulling, sifting or picking at leaf litter on the floor of the exhibit. When the enrichment apparatus was added, other types of foraging were also recorded, such as placing finger, hand or arm in holes of feeders or bamboo pipe, sifting through bran bowl for mealworms and sifting bran in bamboo pipe for mealworms.
Feeding: This was defined by consumption of food. Drinking did not occur very often and was included as 'feeding'.
Locomotion: This behavioural category comprised walking, crawling, climbing, running or jumping movement within the exhibit.
Scanning: Constantly scanning the surrounding environment while sitting or hanging in a resting state.
Affiliation: This was defined as two or more individuals touching each other by allogrooming, leaning against, huddling with, mounting or sitting adjacent (with arm around abdomen) to other animals.
Scent Marking: A behaviour which is often used as an olfactory display of dominance or as a sexual signal (Epple, 1967, cited in Snyder, 1974). This is defined as rubbing circumgenital area or chest (sternal gland) area on objects or substrates (Epple and Lorenz, 1967).
Autogrooming: Consisted of biting at fur, scratching, picking through fur, etc. Tamarins use the hind feet for scratching and the front feet for parting the fur (Snyder, 1974).
Other: Behaviours not falling within the above categories.
Exhibit
Both cotton-top and golden lion exhibits had indoor and outdoor areas, connected by a hole and flap, providing easy access. Each indoor enclosure measured 2.6 m by 2.6 m and was approximately 3 m high, was maintained at a constant temperature of 24°C, and had silk and plastic vegetation, wood branches, a large rock, drinking bowl, feeding bowl, and a nesting box which the tamarins could close themselves; the floor was lined with leaf litter. The outside enclosures contained large branches and living plants and bushes.
Enrichment Apparatus
Feeder Stage: Feeders consisted of PVC Clipsal® junction boxes approximately 7 cm in diameter with two tubular access holes on either side which were approximately 1 cm long. It was thought that the access holes would elicit foraging behaviour that occurs naturally with holes in the wild. Primate cake was placed into the feeders by unscrewing two screws in the lid or by poking into the holes. The intention was that the animals would reach into the feeders to retrieve the food. Three feeders were placed in the cotton-top exhibit and two in the golden lion exhibit; they were carefully wired to branches.
Bran Bowl Stage: Originally a food bowl was used to feed the tamarins; this was reintroduced for the bran bowl stage. The bowl was three- quarters filled with bran and mealworms were added at midday (10 per animal). Bran was considered less harmful than other material such as sawdust if ingestion occurred. It was thought that sifting through the bran would simulate leaf litter foraging.
Bamboo Pipe Stage: One bamboo pipe was placed in each of the exhibits. The pipes were 30 cm long with approximately seven 15 mm diameter holes and twenty 4 mm holes along the sides. At midday the pipe was filled with bran and mealworms (10 per animal). It was intended that the animals would forage with their fingers in the holes and with their arms in the opening at the end of the pipe. One end of the pipe was sealed, the other was left open.
All enrichment devices were placed in the indoor enclosure. The animals were fed at their usual morning, midday and afternoon feeding times. Primate cake was given in the morning and afternoon during all four stages of the study (placed in the feeders only, after baseline observations). Fruit or insects were given at midday for the baseline and , feeder stage, mealworms for the other two stages.
Data Collection
Data were collected from 26 March to 19 May 1993. Haphazard samples (Lehner, 1979) were taken at feeding times. Instantaneous scan sampling (Lehner, 1979) was used to record behaviours, once every minute. Eight sessions totaling ten hours were recorded for baseline observation and for each of the three increasing enrichment stages for each species. The mean session duration was 74 minutes. A total of 80 hours was recorded. Each level of enrichment was conducted over a two-week period. One 'novelty day' was allocated for each new enrichment, during If which data were not collected. The feeders were put in the exhibit first, followed by the addition of the bran bowl, and then of the bamboo pipe. There were two observers, one per species, recording data at the same time.
Analyses
The observers summed the frequency of the behaviours for each session and calculated percentages for time spent in each behavioural category . The Minitab statistical package was used to obtain descriptive and f inferential statistics of the data. The nonparametric Kruskal-Wallis test was used to determine whether differences between treatments (baseline and the three enrichment stages) were significant for individual animals for foraging, scanning, feeding, affiliation and grooming. The data were then transformed by logarithmic transformation and two-way Analysis of Variance used to determine whether foraging and scanning behaviours differed between the species for the four stages.
Results
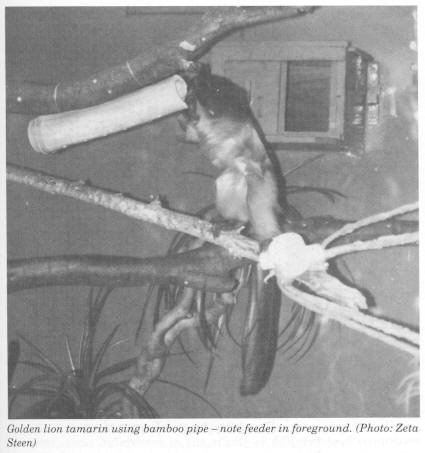
The descriptive statistics for the individual animals for foraging and scanning are displayed in Figs 1-4. Figure 1 displays the mean percentage of time spent foraging for the three cotton-top tamarins for the baseline stage and the three enrichment stages. The cotton-tops all showed a significant increase in the percentage of time spent foraging. There were, however, some differences in the effects of different food acquisition devices. JJ doubled his baseline foraging when the feeder device was implemented (Stage 1). He did not, however, increase foraging time any further after the introduction of the bran bowl or the bamboo pipe. Parr showed a statistically significant increase from baseline (2%) with the addition of the feeders (16%). When the bran bowl was implemented, however, Parr's foraging decreased to 9% .His foraging then increased to 17% during the bamboo stage. Hetti increased percentage of time spent foraging by about 14% during the feeder stage. She did not increase any further with the addition of the bran bowl device. When the bamboo pipe was added, however, Hetti increased foraging time by a further 15%; her foraging percentage peaked at 35% during this stage.
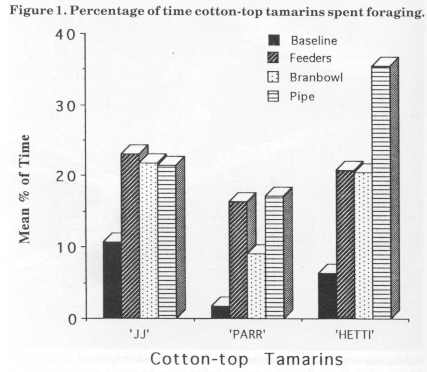
Figure 2 displays foraging percentages for the golden lion tamarins. They both increased foraging with the introduction of the devices. Dye increased by about 10% with the introduction of the feeders, did not increase with the bran bowl and reached a maximum of 20% with the bamboo pipe. Nodye increased baseline foraging percentage time by 15% with the addition of the feeders, by another 4% with the bran bowl and then another 12% with the bamboo pipe. Her foraging peaked at 37% for the bamboo stage.
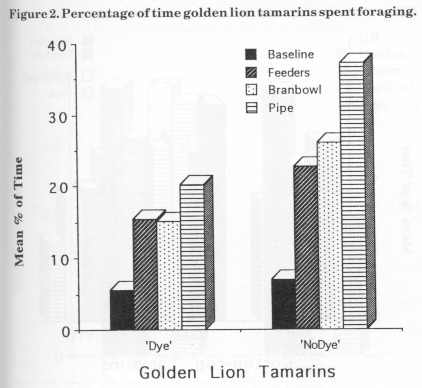
Figure 3 displays the mean percentage of scanning for the cotton-tops for baseline and the three stages of enrichment. The main decrease in JJ's scanning behaviour was observed between baseline (50.5%) and bamboo (33.5%) stage. There was a slight increase (2%) during the bran bowl stage. Parr's scanning also decreased from baseline (61 %) to bamboo (49%) and increased slightly (3%) in the bran bowl stage. Hetti's scanning percentage decreased as each device was added from baseline (46%) to bamboo (22%).
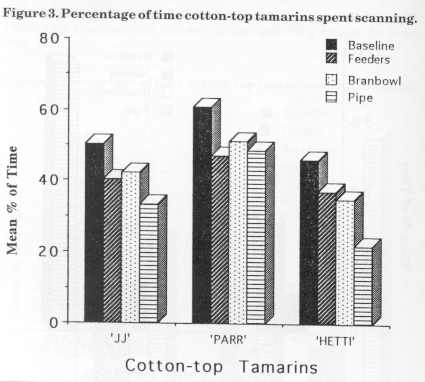
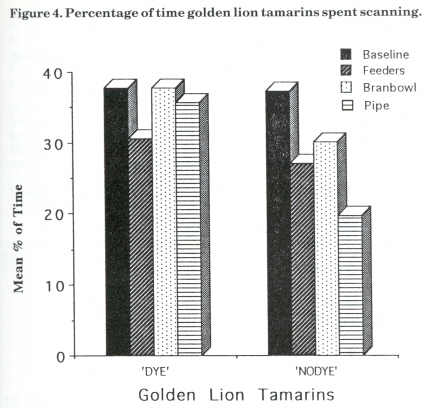
Figure 4 displays the mean percentage of scanning for the golden lion tamarins for the baseline and the three enrichment stages. Dye's scanning decreased from baseline (38%) to feeder (31 %), then increased during bran bowl (38%) and then decreased by 2% in the bamboo stage. Nodye's scanning decreased from baseline (37%) to bamboo (20%) with a slight increase of 3% from feeder to bran bowl stage.
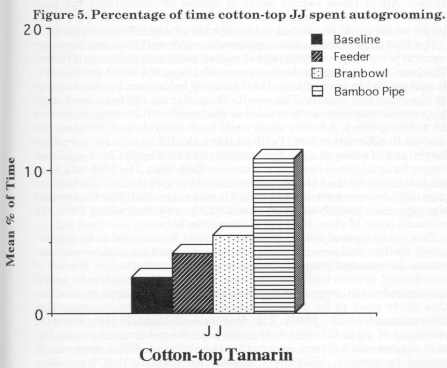
Inferential statistics for foraging and scanning behaviours are given in Table 3 [not reproduced]. Kruskal-Wallis tests showed significant changes in foraging and scanning percentages for all the animals except for Dye's scanning results. Two-way ANOVA tests revealed significant differences between stages for foraging and scanning. A significant difference was found between the species for scanning but not for foraging. No significant differences were found for an interaction between the different species and the different stages. The Kruskal-Wallis test was also used to determine whether there were any differences between treatments for any of the other behaviours for each individual. For JJ there were no significant differences for feeding or affiliation, but a significant difference between treatments for grooming behaviour was found [H= 12.89, df= 3, p < 0.01]. Fig. 5 displays JJ's autogrooming for the four stages. As the number of devices in- creased, autogrooming increased from baseline (2%) to bamboo (11 %). A significant difference was observed for the feeding behaviour of Parr [H = 10.34, df= 3, p < 0.05], but not for affiliation or grooming. No significant differences were revealed for the feeding, affiliation and grooming behaviour of Hetti, Dye and Nodye.
Discussion
(1) Cotton-top tamarins
The aim of the study was to increase foraging time with an increasing number of feeding devices. The cotton-tops all showed a significant increase in the percentage of time spent foraging. There were, however , some differences in the effects of different food acquisition devices. It is possible that JJ's foraging peaked at 22% because he used the bran bowl more than the other two animals. Perhaps the increase in grooming found for JJ could be explained by the fact that during this time he got very dirty (i.e. covered with bran) and then spent more time cleaning himself than he did during the baseline and feeder stages. The main difference in JJ's scanning behaviour was observed between baseline and the pipe. There is not necessarily a relationship between scanning and foraging because of the unexpected grooming result. Although JJ did not increase foraging with the addition of more devices his initial increase was stable.
Parr's decrease during the bran bowl stage could be explained by dominance. The feeder devices were singular, there were a few of them and two animals could forage at one feeder (i.e. two access holes). The bran bowl was big enough for more than one individual to forage at once, however a dominance hierarchy was noticed. The youngest male of the group dominated the bran bowl. Parr spent a lot of time watching JJ at the bowl but would not go near it whilst he was there. Parr did not 294 actually forage at the bran bowl until the fourth session of this stage, and he foraged at the bowl with Hetti. Hetti sometimes waited for JJ to finish at the bowl but at other times joined him. During baseline a hierarchy was noted, there was an order of who retrieved food from the food bowl first. At other times JJ and Parr spent time together in affiliation and autogrooming, and huddled when cold or scared.
Hetti increased percentage of time spent foraging during the feeder stage. Like JJ, she did not increase any further with the addition of the bran bowl. Her peak percentage of foraging at the third stage, when the bamboo pipe was added, was above the estimation made by Redshaw and Mallinson (1991) for foraging by cotton-tops in the wild, but within other estimations for New World monkeys. The other two cotton-tops did reach the wild estimation (25-30%), but were not as high as Hetti. Perhaps there was a gender effect; this could be looked at in a future study with a larger sample size.
(2) Golden lion tamarins
The foraging results for Dye and Nodye also came within the estimation of wild golden lion tamarins' foraging time (Redshaw and Mallinson, 1991). Dye was the subordinate animal and constantly groomed Nodye. She was initially not very active and spent a lot of time scanning and watching Nodye or the public. She showed no significant change in scanning with the addition of the devices. This could be explained by the fact that Dye avoided the apparatus when Nodye was using them. The foraging results for Dye did not, however, appear to affect the amount of time spent scanning or grooming. In contrast to Dye, Nodye increased her foraging from baseline with the addition of each new device; her peak foraging percentage was about 30% more than her baseline one. This is a significant result for the use of food acquisition devices. She also decreased the amount of time spent scanning from baseline to bamboo, another aim of the project.
There were no differences observed between the species for the effects of the devices on foraging. Hetti (C-tt) and Nodye (Glt) both showed similar results. The significant difference found between species for the effect the addition of devices had upon the time spent scanning could be explained by the non-significant result observed for Dye. Considering that there were only two golden lion tamarins, this would definitely affect the mean scanning percentages for this species over the four stages. All of these results must of course be interpreted carefully because of the small sample size. The standard deviations were quite large, which could be related to the number of variables in captivity.
The food acquisition devices appeared to work well in promoting more natural percentages of foraging behaviour, similar to wild counterparts. The bran bowl seemed to be less successful than the other two devices. It still worked in promoting natural foraging behaviour, but not as much as the other two devices. The results found during the bran bowl stage may not have been directly related to the bran bowl. Perhaps there was an ordering effect. A future study could have controls and introduce the devices in different orders. Perhaps there should have been more bran bowls, and of a deeper size. Alternatively sawdust might have produced longer foraging times because it is coarser than bran. The bran may have made it easier for the animals to detect mealworm depletion. The bamboo pipe did however, have bran in it and it was successful. The feeders and the pipe may have been more stimulating, and interesting from the animal's point of view, because of the access holes.
This study showed some significant results which need to be investigated further and are useful for studies on enriching captive environments. Longer and broader studies need to be conducted on the use of enrichment devices with these species, which may ultimately be useful in reintroduction programmes. Enrichment attempts even as small as this study need to be documented to assist in future investigations (Bloomstrand et at., 1986). The feeders and bamboo pipe are good examples of artificial substitutes that promote natural behaviour. Future studies could determine whether the observed effects were real or caused by novelty. Ultimately, studies on animals that have been translocated to naturalistic settings from captivity will show some of the effects of captivity itself (Boice, 1981, cited in Clarke etal.,1982). Ideally, enriching the captive environment of animals should be regarded as essential and not just an optional extra (UFAW, 1990).
[Update: Prior to the study large amounts of fur were found in the nesting box of the golden lion tamarins. The zoo vet suspected that this was a result of overgrooming. During and after the study the overgrooming apparently ceased because the animals had something else to do. After the study was carried out, the zoo introduced an adaptation of the bamboo pipe to the tamarin exhibits. A sturdy loop hook on the roof was used to secure a large swinging branch with access holes drilled along it.]
Acknowledgements
I would like to thank my project partner Ms Jennie McDonnell, our supervisor Dr Rod Wells, Flinders University, and Mr Bruce Campbell, Curator of Mammals at Adelaide Zoo, for advice about the project; the zoo keepers (especially Gert Skipper) and office staff for much time spent accommodating and assisting with project needs. Thanks also to Dr Duncan Mackay for statistics advice, Dr Michael Schwarz, Ms Marianne St. Clair and Mr Gavin Prideaux for advice and comments on the manuscript, and to Dr Schwarz for use of his computer.
References
Bloomstrand, M., Riddle, K., Alford, P., and Maple, T.L. (1986): Objective evaluation of a behavioural enrichment device for captive chimpanzees (Pan troglodytes).Zoo Biology 5: 293-300.
Box, H.O. (1991): Response to environmental change: interrelationships among parameters. In Box, H.O. (Ed.): Primate Responses to Environmental Change (Chap. 3). Chapman and Hall, Cambridge, U.K.
Chalmers, N. (1979): Social Behaviour in Primates. Edward Arnold, London.
Clarke, S.A., Juno, C.J., and Maple, T.L. (1982): Behavioural effects of a change in the physical environment: a pilot study of captive chimpanzees. Zoo Biology 1: 371-380.
Coimbra-Filho, A.F. (1977): Natural shelters of Leorztopithecus rosalia and some ecological implications (Callitrichidae: Primates). In Kleiman, D.G. (Ed.): The Biology and Conservation of the Callitrichidae. Smithsonian Institute Press, Washington, D.C.
Epple, G., and Lorenz, R. (1967): Vorkommen, Morphologie und Funktion der Sternaldrüse bei den Platyrrhini. Folia Primatologica 7: 98-126.
Fleagle, J.G. (1988): Primate Adaptation and Evolution. Academic Press, San Diego.
Forthman Quick, D.L. (1984): An integrative approach to environmental engineering in zoos. Zoo Biology 3: 65-77.
Fragaszy, D.M., and Adams-Curtis, L.E. (1991): Environmental challenges in groups of capuchins. In Box, H.O. (Ed.): Primate Responses to Environmental Change (Chap. 13). Chapman and Hall, Cambridge, U .K.
Garber, P.A. (1992): Vertical clinging, small body size and the evolution of feeding adaptations in the Callitrichidae. American Journal of Physical Anthropology 88: 419-499.
Glatston, A.R., Geivoet-Soeteman, E., Hora-Pecek, E., and van Hooff, A.R.M. (1984): The influence of the zoo environment on social behaviour of groups of cotton-top tamarins (Saguinus oedipus). Zoo Biology 3: 241-253.
Lehner, P.N. (1979): Handbook of Ethological Methods. Garland Press, New York and London. MacDonald, D., ed. (1984): All the World's Animals: Primates (pp. 46-55). Torstar Books, New York and Toronto.
McKenzie, S.M., Chamove, A.S., and Feistner, A.T.C. (1986): Floor coverings and hanging screens alter arboreal monkey behaviour. Zoo Biology 5: 334-348.
Redshaw, M.E., and Mallinson, J.J.C. (1991): Stimulation of natural patterns of behaviour: study with golden lion tamarins and gorillas. In Box, H.O. (Ed.): Primate Responses to Environmental Change (Chap. 12). Chapman and Hall, Cambridge, U .K.
Scott, L. (1991 ): Environmental enrichment for single housed common marmosets. In Box, H.O. (Ed.): Primate Responses to Environmental Change (Chap. 14). Chapman and Hall, Cambridge, U.K.
Sussman, R.W., and Kinzey, W.G. (1984): The ecological role of the Callitrichidae: a review. American Journal of Physical Anthropology 64: 419-449.
Synder, P.A. (1974): Behaviour of Leontopithecus rosalia (golden lion marmoset) and related species: a review. Journal of Human Evolution 3: 109-122.
UFAW (1990): Environmental enrichment: advancing animal care. Video, published by Universities Federation for Animal Welfare, Potters Bar, U.K.
Reproduced with permission of International Zoo News.