I Gilloux J Gurnell1 and D Shepherdson2
1 Queen May and Westfield College, University of London
2 Zoological Society of London, Regent's Park, London
Present address: Metro Washington Park Zoo, 4001 SW Canyon Rd, Portland, Oregon 97221, USA
Abstract
The influence of an environmental enrichment feeding device (puzzle feeder), on activity and behaviour patterns of captive orang-utans, gorillas and chimpanzees was studied at London Zoo. General activity levels and behaviours directed towards the feeder increased for all species when the feeder was filled with food. Chimpanzees used the feeder significantly more (18% of observation periods) than either gorillas (10%) or orang-utans (9.4%).There was considerable individual variation of puzzle use by individuals within each group and time of day also affected use. In some instances abnormal behaviours were reduced. These results are discussed in relation to the management of captive great apes and it is suggested that the use of puzzle feeders can improve the welfare of these animals.
Keywords: animal welfare, environmental enrichment, primates, puzzle feeder
Animal welfare implications
A puzzle feeder offering chimpanzees, orang-utans and gorillas the opportunity of using tools to obtain food items resulted in behavioural changes consistent with improved welfare. For all three species, the feeder resulted in increased feeding related behaviours and more activity. In some cases reductions in abnormal behaviour were documented. No adverse effects were observed. These changes resulted in activity budgets that were closer to those of wild conspecifics. This design of puzzle feeder was simple and cheap to construct, required little additional personnel effort and resulted in significant, positive changes in behaviour for the three ape species. It was, therefore, an effective environmental enrichment tool.
Introduction
Animals housed in zoological gardens are inevitably presented with environments which are impoverished in comparison with their natural ones. Abnormal behaviours such as repetitive locomotion, aggression, fur plucking, auto-aggression and coprophagy have long been associated with captive environments and are often taken as indicators of reduced welfare (Broom 1983, Meyer-Holzapfel 1968, Morris 1964). In general, wild animals live in environments of great spatial and temporal complexity for which they have evolved complex behaviours to enable them to survive and reproduce successfully. It has been suggested that one of the causes of abnormal behaviour in zoo animals may be the lack of opportunity to display a full range of natural behaviour patterns (Hancocks 1980, Hutchins et al 1984, Meyer-Holzapfel 1968). Hughes and Duncan (1988) have argued that animals are strongly motivated to perform some behaviours even in the absence of any necessity, physiological or otherwise, to do so. If this is the case, then deprivation of functional behavioural opportunities may at times be a potential source of frustration.
We consider that the aim of environmental enrichment is to provide a captive environment in which abnormal behaviours are minimized and in which behaviour patterns resemble, as closely as possible, those of wild conspecifics. Aside from sleeping and resting, wild great apes spend most of their time foraging, for example, gorillas 45 per cent (Harcourt & Stewart 1984), orang-utans 46 per cent and chimpanzees 53 per cent (Harvey & Clutton-Brock 1981). Not only does foraging occupy a large amount of time for wild apes but it may also be a source of intellectual stimulation as various studies of tool use by wild chimpanzees would suggest (Beck 1980). In captivity apes spend very little time foraging because their food is presented in a way which requires minimal intellectual or manipulative skill to obtain and consume. Several studies have demonstrated a link between increased foraging and reduced abnormal behaviour (Anderson & Chamove 1984, Bloomsmith et al 1988, Daman 1990, Gould & Bress 1986).
Ever since Jane Goodall's pioneering studies on the chimpanzees of the Gombe Stream Reserve (Goodall 1968) it has been known that these animals will make and use simple tools to obtain food. Many zoos have attempted to create similar tool using opportunities for their chimpanzees by providing artificial 'termite mounds' and giving the animals sticks to use as tools to obtain food rewards, such as jam and yoghurt, from within the 'mounds' (Dow 1986, Nash 1982, Poulsen 1975, Shepherdson 1988). Less well documented are the tool using abilities of the other great apes. Although not observed in the wild, in captivity both gorillas and orang-utans will use tools to obtain food and several authors have described puzzle feeders for gorillas (Boysen & Frisch 1987, Cole 1987, Natale et al 1988, Wood 1988) and orang-utans (Murphy 1976, Seymour & Shepherdson 1991).
In this study we evaluate a simple puzzle feeder as an enrichment device and compare its effectiveness for each of the great ape groups at London Zoo.
Materials and methods
Three groups of great apes were studied. The first was a pair of orang-utans (Pongo pygmaeus pygmaeus): a mature male and an adult female; the second a group of four gorillas (Gorilla gorilla gorilla): a mature male silverback, two mature females and a juvenile, and the third a group of seven chimpanzees (Pan troglodytes) comprising a mature male, four mature females and two juveniles. Each group was housed in its normal cage (Toovey & Brambell 1976) consisting of a den and an outside enclosure.
The feeder (Figure 1) in each cage consisted of an open-ended 3m length of 15cm diameter plastic drain pipe attached horizontally to the outside of the enclosure weldmesh. An opening in the back of the pipe, away from the animals, allowed the middle to be filled with food items. The apes could manipulate food items to the end of the pipe by inserting sticks through holes drilled along the side of the pipe facing them. Once the food was at the end, the animals could reach it with their hands. The sticks were either bamboo canes or willow twigs 40cm to 70cm long; between 6 and 10 were placed in the cage at any one time in order to ensure that a surplus was always available. Food placed in the feeder at the beginning of a trial consisted of a mixture of 10 different fruits (apples, bananas, dates, grapefruit, melons, oranges, plums, raisins, sultanas and tomatoes) plus celery, carrots and biscuits.
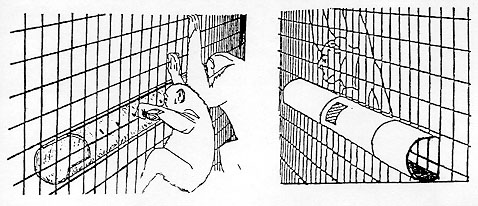
Observations were carried out on dry days during the summer months, July-September, of 1988. Each group was studied for 12 two-hour trials without food in the feeder, six in the morning between 0930h and 1130h and six in the afternoon between 1430h and 1630h, and 12 two-hour trials with food in the feeder, six in the morning and six in the afternoon.
An instantaneous, scan sampling procedure was adopted (Martin & Bateson 1986). This involved the observer scanning the group at one minute intervals and recording the behaviour of each member of the group at that time. Thus, within each two-hour trial, 120 records of the behaviour of each animal were obtained. Nineteen species- specific behaviour elements (Table 1) were recorded on an Epson HX-20 portable microcomputer.
For statistical analysis, the behaviour elements were classified into five mutually exclusive categories: abnormal, feeder-oriented, non-active, active and interactive (Table 1).

The category 'abnormal behaviour' included coprophagy, regurgitation and reingestion. The absence of any observations of regurgitation and reingestion in the wild suggests that it is an abnormal behaviour (Gould & Bress 1986). Coprophagy has been seen in wild mountain gorillas (Harcourt & Stewart 1978) but is rare and only occurs during periods of poor weather. Coprophagy is, therefore, not strictly an abnormal behaviour, at least
for gorillas. It is however, undesirable in captive apes because of the potential for disease transmission.
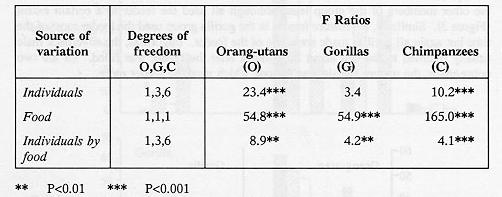
The proportion of behaviour in each category was converted using an angular (arcsine) transformation and then subjected to analysis of variance (ANOVA) to test specific hypotheses about the behaviour of the animals in each group with and without food in the feeder. Because many comparisons have been made in these tests, we have adopted a significance level of one per cent for a type I error. Three analyses will be presented here:
1. The effects of the presence of food in the feeder on each behaviour category of the animals in each group during the two-hour trials.
2. The effects of the presence of food in the feeder on feeder-oriented behaviour of each individual in each group during the two-hour trials.
3. The effects of the presence of food in the feeder on each category of behaviour of the animals in each group during the first 30 minutes of the two-hour trials. This analysis was carried out because it was observed that the food in the feeder often ran out before the end of a two-hour trial and this could lessen the effects of the feeder on the behaviour of the animals judged over a whole two-hour period.
Another factor, whether the trials were carried out in the morning or afternoon, was included in an analysis of variance, and although the results of these tests will not be presented, some of the findings will be referred to in the discussion. Since the number of individuals and group structure varied among the orang-utan, gorilla and chimpanzee groups, we decided that adding 'species' as a factor to the ANOVA analyses would make the interpretation of the results over complicated and indeed, inappropriate. However, clear differences in behaviour between the species are discussed below.
Results
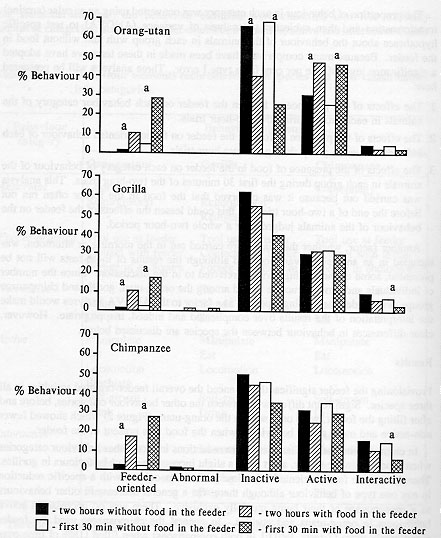
Provisioning the feeder significantly enhanced the overall feeder-oriented activities in all three species. Significant differences between the other behaviour categories, before and after filling the feeder, were only seen in the orang-utans (Figure 2) which showed fewer non-active and more active behaviours when the food was present in the feeder.
In chimpanzees and gorillas there were reductions in most other behaviour categories when food was in the feeder, apart from a slight increase in active behaviours in gorillas. Therefore, more feeder-oriented behaviour was not associated with a specific reduction in any one type of behaviour although there was a general decrease in other behaviours as a consequence of their being mutually exclusive. There was no decrease in active behaviours in orang-utans and gorillas. In general the chimpanzees used the feeder significantly more (Z tests, P < 0.01 in both comparisons) when filled (18% of time over two hours) than either gorillas (10%) or orang-utans (9.4%).
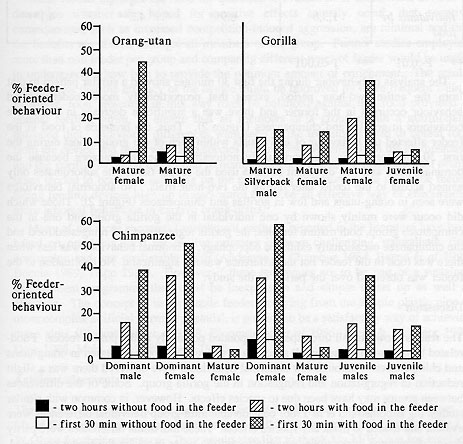
There were differences in the response of different individuals in each group to the presence of food in the feeder over the two-hour trial periods (see Table 2). For example, one of the mature female chimpanzees used the feeder most and interacted with the other members of the group least, although all used the feeder to a certain extent (Figure 3). Similarly, the mature female in the gorilla group used the feeder most of the time but again all gorillas made some use of the feeder. In gorillas, the silverback male usually returned to the den about 20 minutes after the feeder was filled. Of the two orang-utans, the mature female was the one which used the feeder more.
The analysis of behaviouir during the first 30 minutes showed a similar pattern to that from the entire two-hour period, except that proportionally more feeder-oriented behaviour occurred in the former and there was a significant decrease in interactive behaviours in gorillas and chimpanzee (Figure 2). Thus, the presence of food in the feeder affected the behaviour of individuals within each of the groups most during the first 30 minutes. This was particularly noticeable in the chimpanzees because the dominant male and two dominant females used the feeder first. The subordinate only gained access to the feeder later during the two-hour trials. No abnormal behaviours were seen in orang-utans and few in gorillas and chimpanzees (Figure 2). Those which did occur were mainly shown by one individual in the gorilla group and one in the chimpanzee group, both mature females; the gorilla regurgitated and reingested food and the chimpanzee occasionally exhibited coprophagy. Abnormal behaviour was less when there was food in the feeder but the difference was not significant. No habituation to the feeder was observed over the period of the study.
Discussion
The results show that all three species responded positively to the puzzle feeder. Food-related behaviour increased in all species and active behaviours increased in orang-utans and chimpanzees. Although few abnormal behaviours were observed there was a slight reduction in regurgitation and reingestion in the gorilla group. Some of the differences between groups may have been due to species effects. However, in common with similar studies (eg Bloomstrand et al 1986) considerable intra-group individual differences were evident and tended to mask any species effects. These individual differences were partly due to dominance interactions, which affected the order in which the feeder was used, and also to individual preferences - some animals appeared to be more attracted to the task than others.
Some differences in feeder-oriented behaviour between morning and afternoon trials occurred, especially in gorillas. These animals tended to be less active and interact less in the afternoon when the silverback male was in the den. One of the mature females often remained in the outside enclosure because the other female prevented her from entering the den. Behaviour of the gorillas was not affected by whether the feeder was filled with food. The female orang-utan was not very active in the afternoon when there was no food in the feeder. The effect of food in the feeder in the afternoon was most pronounced in the case of the chimpanzees. In the morning, the dens were locked - this was not so in the afternoon when dominant individuals used them some of the time, thus allowing the others ready access to the feeders
These results highlight the need for quantitative evaluation of enrichment devices to determine whether the hoped for positive effects actually occur, that negative consequences, such as increased competition-induced aggression, are minimal and that the benefits are shared amongst all members of the group. Further studies employing more than one feeder per group and comparing different types of feeder would be useful in understanding how best to provide the optimum amount of enrichment. The results also suggest that the efficacy of the device might be improved by more frequent filling. However, food presented in this way must be balanced against that food provided at normal feeding times, to ensure that all members of a group obtain a nutritionally balanced diet. Individual differences were seen in the techniques used to obtain food from the device and there were also species differences. Whereas both the chimpanzees and the gorillas stood on the ground to manipulate food items, the more arboreal orangutans tended to brachiate and hang on the side of the enclosure while using the feeder. Siting feeding devices high up in enclosures could be an effective way of encouraging more arboreal behaviour in captive orang-utans.
Many of the more successful enrichment methods reported in the literature have been based on providing food for captive primates in more challenging, naturalistic, time consuming and less predictable ways (Anderson & Chamove 1984, Bayne et al 1992, Boccia 1989, Tripp 1985). However if these techniques are to be part of a sustained management programme they must be inexpensive and simple to set up as well as effective. The concept of using puzzle feeders, ranging from the simple plastic pipe to more complex artificial 'termite mounds', is proving to be a satisfactory way of achieving these aims (Bloomsmith et al 1988, Bloomstrand et al 1986, Brent & Eichberg 1991, Dow 1986, Maki et al 1989, Nash 1982).
Acknowledgements
The authors are grateful to the Zoological Society of London for providing facilities, to Mr M Carman and the keeping staff in the Sobell Pavilion, to Mr P J Olney and Dr J H W Gipps for their assistance. They would also like to thank Ms M Walton for graphics, Mr G Ray for technical assistance, Dr A Peace for statistical advice and Dr J Mellen for commenting on the manuscript.
References
Anderson J R, Chamove A S 1984 Allowing captive primates to forage. In Standards in Laboratory Animal Management, pp 253-256. Proceedings of a Symposium. Universities Federation for Animal Welfare: Potters Bar
Bayne K, Dexter S, Mainzer H, McCully C, Campbell G, Yaxnada F 1992 The use of artificial turf as a foraging substrate for individually housed rhesus monkeys (Macaca mulatta). Animal Welfare 1: 39-53
Beck B 1980 Animal Tool Use. Garland STPM Press: New York
Bloomsmith M A, Alford P L, Maple T L 1988 Successful feeding enrichment for captive chimpanzees. American Journal of Primatology 16: 155-164
Bloomstrand K, Riddle K, Alford P, Maple T L 1986 Objective evaluation of a behavioral enrichment device for captive chimpanzees. Zoo Biology 5: 293-300
Boccia M L 1989 Preliminary report on the use of a natural foraging task to reduce aggression and stereotypies in socially housed pigtailed macaques. Laboratory Primate Newsletter 28(1): 3-4
Boysen S, Frisch D 1987 Extractive tool use in captive lowland gorillas. American Journal of Primalology 12: 332
Brent L, Eichberg J W 1991 Primate puzzleboard: a simple environmental enrichment device for captive chimpanzees. Zoo Biology 10: 353-360
Broom D M 1983 Stereotypies as animal welfare indicators. Current Topics in Veterinary Medicine and Animal Science 23: 81-87
Cole M 1987 How we keep our gorillas occupied. Proceedings of the 13th National Conference of the American Association of Zoo Keepers. Animal Keeper's Forum (special edition): 401-403
Daman F J 1990 Effects of the addition of browse on the feeding behaviour (time budget and coprophagy) in captive bonobos. Scientijlc Papers, 45th IUDZG Conference. Copenhagen Zoo: Denmark 180-182
Dow S M 1986 Behavioural enrichment for captive animals. Proceedings of the 25th Anniversary Symposium of the British Veterinary Zoological Society: 67-77
Goodall J 1968 The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Animal Behaviour Monographs 1: 165-311
Gould E, Bress M 1986 Regurgitation and reingestion in captive goriallas: description and intervention. Zoo Biology 5: 241-250
Hancocks D 1980 Bringing nature into the zoo: inexpensive solutions for zoo environments. International Journal for the Study of Animal Problems 1: 170-177
Harcourt A H, Stewart K J 1978 Coprophagy by wild mountain gorilla. African Wildlife Journal 16: 223- 225
Harcourt A H, Stewart K J 1984 Gorillas time feeding: aspects of methodology, body size, competition and diet. African Journal of Ecology 22: 207-215
Harvey P H, Clutton-Brock T H 1981 Primate home-range size and metabolic needs. Behavioral Ecology and Sociobiology 8: 151-155
Hughes B 0, Duncan I J H 1988 The notion of ethological 'needs', models of motivation and animal welfare. Animal Behaviour 36.- 1696-1707
Hutchins M, Hancocks D, Crockett C 1984 Naturalistic solutions to the behavioural. problems of captive animals. Zoologischer Garten 54. 28-42
Maki S, Alford P L, Bloomsmith M A, Franklin J 1989 Food puzzle device simulating termite fishing for captive chimpanzees (Pan troglodytes). American Journal of Primatology Supplement 1: 71-78
Martin P, Bateson P 1986 Measuring Behaviour. Cambridge University Press: Cambridge
Meyer-Holzapfel M 1968 Abnormal behavior in zoo animals. In Fox MW (ed) Abnormal Behavior in Animals, pp 476-503. W B Saunders: Philadelphia
Morris D 1964 The response of animals to a restrained environment. Symposium of the Zoological Society of London 13: 99-118
Murphy D E 1976 Enrichment device for orangutans and chimpanzees. International Zoo News 23(137): 24-26
Nash V J 1982 Tool use by captive chimpanzees at an artifical termite mound. Zoo Biology 1: 211-221
Natale F, Poti P, Spinozzi G 1988 Development of tool use in a macaque and a gorilla. Primates 29(3): 413-416
Poulsen H 1975 Keeping chimpanzees occupied in captivity. International Zoo News 22 (1): 19-20
Seymour S, Shepherdson D J 1991 Puzzle Feeder for Orang utans. Environmental Enrichment Report 3. Universities Federation for Animal Welfare: Potters Bar
Shepherdson D J 1988 The application of behavioural enrichment in zoos. Primate Report No 22: 35-42
Toovey J, Brambell M 1976 The Michael Sobell pavilions for apes and monkeys. International Zoo Yearbook 16: 212-217
Tripp J K 1985 Increasing activity in captive orangutans: provision of manipulable and edible materials. Zoo Biology 4.- 225-234
Wood R J 1988 Spontaneous use of sticks as tools by gorillas at Howletts Zoo Park, England. International Zoo News 35 (185): 13-18
This article originally appeared in Animal Welfare 1: 279-289 (1992)
Reprinted with permission of the publisher.