ARNOLD S. CHAMOVE
Stifling University Psychology Department
Stifling FK9 4LA Scotland
SUMMARY
This review supports the idea that enrichment is an attempt to ameliorate problems caused by containment, that the goals of enrichment are to alter behaviour so that it is within the range of the animals' normal behaviour, and that evaluation of the success of enrichment techniques is important. I suggest that the idea of 'increasing psychological space' can act as a unifying concept for many of the techniques of enrichment, that is, many of the successful enrichment techniques act in a way similar to that of increasing physical space.
Recently there is increasing research interest in improving the welfare of captive animals, but there has been less interest in the rigorous operational definition of terms used in the description of environmental 'improvement' or enrichment, in evaluating the cost-effectiveness of enrichment, or in the development of theoretical models that guide research and integrate divergent studies. There are exceptions. [28,32,33,48,89,111,138,148]
The study of enrichment may be viewed as a conceptual extension of past research investigating the effects of early experience on the development of behaviour. And in these studies there are many suggestions about the directions in which enrichment research might fruitfully go. The use of deprivation or restriction was designed to answer questions such as: What is the role or importance of a given aspect (for instance, vision) in the expression of social interaction or in social development? In these studies, experimenters deliberately removed or restricted the variable in question; for example, by observing subjects during brief periods in the dark, either humans [74] or monkeys, [7,27] and by depriving animals for longer periods of sight [11,122] or of particular types of visual input; [132,135] and they observed blind humans naturally deprived of vision. [68,90] In enrichment studies similar questions are addressed, but now by observing individuals in relatively impoverished environments and subsequently supplying the variable to the already deprived individual in order to assess its importance for behaviour. There are often two goals of enrichment studies: to improve the captive environment and to assess the importance of a given aspect for the animal's behaviour, both achieved by additions to an impoverished environment which is at present accepted as the norm.
The aims of this contribution are to look at three areas which are addressed by enrichment studies, within the context of a review: (1) the problems produced by spatial restriction, (2) the goals of enrichment, and (3) the evaluation of enrichment techniques. I hope to put the first two of these into a conceptual framework to help guide future research.
PROBLEMS OF CONTAINMENT
The goals of enrichment may be difficult to achieve because of certain obstacles caused by the nature of containment. The basic problem is that caged animals cannot or do not carry out their normal range of behaviour.
Cage size
One fundamental reason that captive animals are behaviourally restricted is simply lack of space; the enclosure is too small. There is evidence that too small cage size is powerful enough to even inhibit that most robust measure of general well-being, namely that of reproduction. [51,138] The mechanism for this inhibition is most likely to be the resulting adrenocortical stress response.92 Many behaviour patterns are influenced by cage size and its related variable of crowding [1,8,45,56,93,138,145] and group size. [39,77,110] Of course group size even has effects on behavior in the wild where spatial restrictions are less salient or even absent. [71,97,101,106,137]
It is widely accepted that small cages increase the incidence of stereotyped movements and other non-locomotory abnormal behaviour. [31,32,52,120] It is hard to imagine a case where simply increasing usable cage space would not constitute an enrichment procedure (but some disagree [154]), even though additional increases of large enclosures might not dramatically change certain behaviour patterns, such as gross activity levels or inter-individual distance. It is likely that the ratio of benefit relative to cost would rapidly decrease after a critical cage size had been exceeded. The level at which increases in cage size would lead to substantial changes in behaviour could serve as an objective guide to minimal cage sizes for any given species. Of course these critical levels are different for different behaviour patterns. [145] For example, one might expect activity stereotypes to disappear with a smaller increase in-cage size than expected for exaggerated levels of aggression, for excess self-directed behaviour, and for behaviour caused by the absence of opportunities for foraging. [36]
The literature on the effects of crowding [8,36,48] shows that, surprisingly, it is the number of individuals in an enclosure which is the important variable and not the space available for each individual. Of course it is likely that this holds true only within certain limits; and in very large enclosures which are larger than the range of the animal or in very small enclosures where personal space is constantly violated (for example, battery cages), other mechanisms come into force.
While there are many questions about environmental enrichment with theoretical relevance, we can also ask at a predominantly practical level how we can improve enclosures.
Increasing psychological space
The problem with cage design is that it often does not make use of available space. Another way of counteracting deficiencies in cage size is by increasing the 'psychological space' of the enclosure. This can act as a unifying idea for many of the non-social techniques of enrichment.
To choose what aspects of the enclosure need to be changed to increase psychological space, one must understand what aspects of space are important to the animal in question. In other words, what do individuals do with additional space when they have it? They move through it, using it for various activities; they use the space to avoid other individuals and predators; they use it to look for food; et cetera. There are at least three ways to increase psychological space. The simplest is the better use of existing space such as walls, floors, ceilings, and of course the cage interior.
Walls
Related to the problem of cage size is that of cage design. Cages and enclosures are commonly designed by architects and their primary considerations are those of engineering, maintenance, cost, and human comfort. Bare, flat walls are easy to construct and to keep clean, but many animals cannot use flat walls. Often in zoos one sees primates housed in spacious cages, but in ones in which large open spaces go unused by the animals; and one problem of enrichment is how to use these valuable areas most efficiently. [12] One recent study we have completed [38] is illustrated in Figure 1. To assess the effects of vegetation on callitrichid monkey behaviour , climbing plants were grown on three types of trellis fixed about 10 cm from smooth walls. Plants grew best on the small-mesh flexible trellis, while monkeys spent most time on the large-mesh rigid trellis. The presence of plants had no measurable effect on monkey behaviour, but the presence of the trellises did enrich the environment by allowing the monkeys to make better use of the available space.

Floor
The floor is one area of the cage that which is often ill-designed or neglected. The floor and areas just above the floor are often kept clear for easy cleaning. In the large (27m3) enclosure that we use for callitrichid monkeys, the floor makes up about 40 % of the total surface area that monkeys can use and as much as 66% of the horizontal surface area. In captivity these same monkeys almost never visit the floor (1% of the time) when it is bare but do so more frequently (15% of the time) when the ground is covered with some leaf-like substrate.110 In the wild these arboreal monkeys occasionally come to the ground to forage in the leaf litter or to cross open areas. [85,116,141]
The effect with terrestrial monkeys is even more dramatic. In a zoo, groups of ground living primates (Lemur catta, Cercopithecus aethiops, Macaca arctoides) spent only about 15% of the day on the floor when it was bare and dirtied with faeces and urine as normal. When the floor was covered with woodchips, time spent on the floor increased to over 70%, a more appropriate use of the cage space [6,30,34] and closely approximating the proportion of horizontal surface area it occupied in those cages and to wild patterns. Allowing domesticated animals the opportunity to forage has also led to reductions of abnormal behaviour and increases in activity levels (for example, chickens, [9,87] pigs, [55,99,146] cows [4]).
Interior
Yet another aspect of primate cage design which has been virtually ignored is that of what goes into the interior of the cage. In laboratories this area is often empty or has a simple metal shelf, but some researchers have systematically offered their macaques branches. [123,125] With smaller monkeys the interior area may be filled with branches. [38,62] One field study suggests that callitrichid monkeys primarily use horizontal branches, and they rarely (4%) move on vertical supports, mainly large tree trunks,72 Nevertheless, many of the cages used for these monkeys have only a few rigid horizontal branches with many thin flexible vertical branches hanging from them. Perhaps this is because it is easy to hang and replace these vertical clumps. Measurements in our colony indicate that about 25% of the branches are horizontal, 30% oblique, and about 45% vertical, with vertical branches much thinner than horizontal ones. In the wild, thin vertical branches are used almost exclusively for access to food, as illustrated in Figure 2. In the wild [70] and in very large outdoor areas the monkeys spend most of their time (89%) [40] in dense networks of thin, flexible, non-woody tangles. These are not normally found in captive situations.

We are currently looking at branch use and branch preferences in caged callitrichid monkeys.38 From our preliminary results it is clear that these monkeys prefer a large number of unevenly spaced branches; widely spaced supports are especially used by the more active juveniles and narrowly spaced ones by the less active adults. However, it is well known that the preference of animals is not necessarily in their best interest in captivity. [2,23,46,47,53,54,87,88,109]
Another way to better use of existing space is by sharing space. This can be done by mixing species, by making use of their characteristic use of different areas and that they do not react to other species as they do to their own. Mixing species also enables two enclosures to be combined giving more space for each species. [118,147] Ground living rodents eat fallen fruit in bird, bat, or primate exhibits in Edinburgh, Auckland, and Milwaukee Zoos. The presence of other animals also increases the complexity of the environment, although this aspect of enrichment has only been touched upon.96 C. cephus and C. nictitans are two monkey species often (80%) seen in polyspecific associations in the wild.
While animals can share the same space at the same time, they can also share space sequentially. Techniques employing the rotation of animals are rarely reported. If animals are very active for only 50% of the day, they could be allocated an exercise area for half the day. [62] For certain scent-marking animals, this may make the area more interesting, for others more threatening.108 Longer-term rotation is also a possibility with different species. If enclosure design permits, moving a carnivore from its enclosure would allow a herbivore access to an area with long grass and recovering saplings. It is difficult to understand why such a procedure is not regularly used in zoos.
Dividing
Dividing existing space is another technique of increasing psychological space. [60,61,109] This technique seems especially effective in reducing aggression, probably because visual input is so important for that behaviour. [27] Dividers can be permanently in place [25] or only used during periods of competition, like feeding time.[10] Aggressive competition in the presence of food, commonly offered in only one or two discrete locations, may be increased over non-feeding conflict by a factor of 10 with a concomitant 50% increase in blood cortisol levels, [6,37] suggesting considerable stress. Hiding food [25,131] or making it more difficult to process by freezing it [6] can reduce the competition.
The use of dividers can also require the animal to cover more distance when moving from one area to another. In a study just completed, we divided rectangular rodent cages into a maze using strips of plastic. Several developmental changes in the behaviour of the inhabitants resulted, especially an increase in activity and lower emotionality. [23]
Foraging
One can increase psychological space both by encouraging animals to make more use of existing space and by changing existing space used. If individuals have to look for hidden food, then that space is changed in that it contains more bits of information, has more potentially relevant choices. The most effective technique so far developed is to encourage foraging by scattering small items of a desirable food into the floorcovering. Currently we are looking at other techniques for arboreal monkeys involving foraging in holes (see Figure 3).

Learning
Individuals perform various other activities in large spaces. They learn the characteristics of their environment to enable them to find food, to threaten and avoid conspecifics, and to flee from predators. They approach novel stimuli even though these are frightening, in order to learn about them. [110] They seek out these challenges to reduce their fear of novelty in the future. If one of the activities that go on in large complicated spaces is that of learning, then we can set tasks that require learning and see if they serve as enrichment, and serve to increase psychological space. One way that we are experimenting with this is by making small, regular changes in the positions of branches in enclosures.
Energy
Calorie expenditure is another by-product of increasing space. Many of the tasks devised by Markowitz [103,104] require subjects to walk or run from one operant device to another. There is evidence that calorie expenditure improves mood in humans, [28,65] and it is likely that it will do so in caged animals when rigourously tested. One might expect it to be more important in those species which commonly expend more energy.
Variability
Increased space requires a corresponding increase in the variability of behaviour, therefore any increase in variability requirements or possibilities would be expected to act similar to increasing psychological space, and therefore be enriching. We are aware that different species are 'prepared' to do different things. Callitrichids use objects in their play, macaques also play with objects, capuchins also use tools, chimpanzees also make tools. When we gave tamarins a wheel device to run and play on, which had proved effective with macaques, [82] they neither used it nor played with it (see Figure 4). When the preparedness of chimpanzees to make and use tools was challenged (see Figure 5), they extensively used the device they were given. [29] Making ropes more variable and less predictable led to increased use.
GOALS OF ENRICHMENT
Two complementary short-term aims of enrichment studies are to increase 'desirable' behaviour and to reduce 'undesirable' behaviour. It is widely accepted that activities such as coprophagy, regurgitation, hair-pulling, self-injury, or stereotyped movements are undesirable. Conversely exploration, play, affiliation, and foraging are commonly considered desirable. Other behaviour may be classed as one or the other depending on its frequency: withdrawal or avoidance, reproductive behaviour , aggression, inactivity, displacement, and feeding are desirable if moderate in frequency but usually undesirable if very frequent. [14,35,78,79,128,142] For example, overeating, hyperaggression, and long periods of inactivity are believed to be indicative of poor health. Furthermore, an increase in desirable behaviour is usually believed to be associated with an improvement in physical or psychological health. [67]
There are at least three approaches in assessing desirability of behaviour: normality, observer/caretaker acceptability, and theoretical considerations. Each may sometimes be conceived independently, but they are often related and lead to similar solutions. Probably the most common approach emphasizes normality: Behaviour which approximates that found in the wild is held to be desirable. In other words, behaviour should fall within the range of values for the form and frequency seen in the wild [107] (but see Wemelsfelder [148] p.137). One problem of implementation is that the norms for many behaviour patterns vary, sometimes widely, with group size, habitat, season, and even from day to day with local weather conditions. [41,83, 117, 129] If callitrichids rarely come to the ground in the wild, possibly because of predator pressure,85 is it normal/desirable that they do so in captivity and so better use the available space? It is not normal for animals to operate mechanical puzzles in the wild, but is it desirable for them to do so and thereby keep active in captivity? Sign language in the great apes is another example illustrating that enrichment does not necessarily directly lead to normal behaviour, although enrichment often leads to more normal levels of other behaviour patterns.
The second approach takes into account what is acceptable to keepers, technicians, and the public in zoos. The last is reported to like active and easily visible animals; [1,42] but they do not like to see certain normal behaviour like mating, and dislike viewing certain abnormal behaviour patterns, such as regurgitation, reingestion, and coprophagy (shown by some captive gorillas [3, 79]) .When manipulable floor-coverings with food-items led to increased activity in zoo-living orangutans, the exhibit was rated more favourable by visitors. [142] The mismatch between the public preferring animals to be visible and the animals' possible preference to be hidden by foliage, can be reconciled by education. [155]
Theoretical
The third approach is to choose enrichment goals because they are identified from a theoretical rationale. This rationale might be to occupy the animals, to exercise them, or to increase more normal behaviours. An example might be a goal of inducing more exercise or calorie expenditure for animals, based on their normal, free-ranging, or wild activity budgets (discussed in Chamove [28]), or increasing problem solving opportunities, for example through burying food in woodchips, [33] while taking into account feeding techniques in the wild. The use of swimming and fishing pools are other innovative examples requiring effort or skill by laboratory primates. [75,96]
I have argued elsewhere [113] that the goal of enrichment should be to allow and encourage animals to show behaviour patterns which are within the normal range of their wild counterparts. Others [138] have suggested the goal should be to produce animals which could survive and reproduce if released into the wild.
Stress
The reduction of stress is another target of some enrichment studies. Poor cage design, cramped cages, crowding, et cetera are believed to stress animals, and as described above, a few studies have associated cage characteristics with physiological and behavioural measures of stress. [18,43,51,61] I suggest that the problem actually is to achieve the optimal level of stress or arousal rather than just trying to reduce it to the lowest level possible. In the normal wild animal, the levels of arousal vary with the normal challenges of life (for example, Beuving [13]). This suggests that any optimal level is (a) above zero, (b) is a variable one, and most importantly (c) that the duration of the peaks of arousal is brief.
The avoidance of stress illustrates that 'stress' has come to be viewed as equivalent to 'distress'. Garmezy [73] defines stress as 'any action or situation that places special physical or psychological demands upon a person—anything that serves to unbalance an individual's equilibrium or homeostasis' (p.238). If stress is any event that destabilizes and taxes the system, then (a) positive events are also of interest, and (b) some stress is not necessarily undesirable. In this discussion I will use the term 'arousal' to refer to the consequences of both positive and negative events, and 'stress' to refer to the long-term consequences of negative events.
Both the human and animal literature suggest that levels of stress or arousal which are markedly and persistently lower than those likely to be found in the wild lead to individuals who do not adapt well to subsequent stressors or adapt well even in the absence of identified external stressors. They over-react both in intensity and duration to mild stimuli and stressors (for example, [49,140]). It appears as though they have difficulty inhibiting a response once a response has become probable.26,133 Some stress early in life appears to help individuals to cope with stressors when they are older. [19,24,49,50,63,87] Also adult animals appear to seek challenge when living in captivity. [6,22,81,84,91,94,103,104,105,110,115,150] A growing number of studies have shown that animals may choose to work for food despite the free availability of the same food. We are currently trying to determine why callitrichid monkeys select peanuts in their shells (80% of choices) to those already shelled even though the former take more effort.
We have just completed a study which strongly suggests that brief arousal is beneficial to animals living in the constant conditions of a laboratory. During days on which occurred arousal caused by the normal husbandry procedures of capture of infants or adults, or when large bird-like silhouettes passed over the enclosures, we found that monkeys' behaviour was more like that seen in enrichment studies than that seen in long-term stress studies. [113] This similarity was less evident in the morning evaluation taken immediately after the arousal than in the afternoon. The data is presented in Table 1 and shows that six behaviour patterns (in bold) can be used to differentiate beneficial effects of enrichment from those of long-term stress.
One abnormal behaviour which appears to be a good indicator of a poor environment is stereotypy. It is often suggested that the presence of repetitive stereotyped movements 'can be seen as the animal's attempt to increase its sensory input' [9,53,148 p.143]. 'To increase sensory input' implies an existing low level of input which needs to be increased in either the amplitude of stimulation, an increase in the variability of stimulation, or a change in the particular stimulation being experienced.
Stereotyped movements appear to occur when there is some arousal or stress. This may be due to boredom, frustration, fear, or merely stimulus change. [12,31,36,120] It seems more likely that stereotyped movements are habitual patterns of stimulation which, because of their familiarity and repetitive nature, are calming to the individual. Stereotyped behaviour is followed by lower adrenocortical levels. [5,14,45] The patterns are developed through long periods of deprivation or restriction and are therefore resistant to change (discussed elsewhere [5]). The reduction of stereotypy is a sensitive measure of enrichment. [21]
Table 1
Significant changes (non-significant changes are in brackets) from baseline in behaviour from five studies of enrichment, from the present study, and from three studies involving stress.
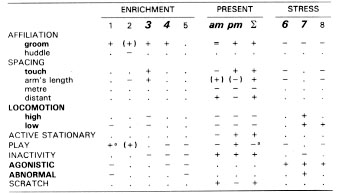
(1) Boccia, 1989; [17] (2) Westergaard & Fragasgy, 1985; [149] (3) McKenzie et al., 1986 [109] (4) Chamove et al., 1982 [34] (5) Anderson & Chamove, 1984 [6] (6) Glatston et al., 1984 [76] (7) Chamove et al., 1988 [39] (8) Worsley-White, 1988 [152]
Range of behaviour
Cages also function to limit the range of behaviour shown by an animal. Several lines of research have shown that increasing the range of foods eaten is more healthy [98] increasing the range of muscles used is healthier,144 and increasing the range of stimuli to which one is exposed is healthier [18,55,67,80,95,113,127,130] and less boring. [148] Consequently, enrichment studies can try to increase the range of behaviour expressed [55] while increasing the range of stimulation available. [121]
The most obvious of these behaviours, and considered by some to be the most important [69,119] is the opportunity to move and forage. Primates spend between 25% and 90% (mode = 55%) of their day searching for and processing food. [44] Even in large semi-natural enclosures where monkeys are given food in excess of their needs, mandrills still spend 45% of the day foraging (Feistner, pers. com, 2/88). In cages this is dramatically reduced, to about 4% in callitrichids (personal data), 5% in chimpanzees and gorillas,16,79 and to 6% in macaques. [6,17] Techniques to increase the search or processing time can increase this to around 10%, 20%, and 30% respectively.
Other attempts to encourage foraging have not been as successful. Nash [114] evaluated an artificial termite mound where chimpanzees could fish for mashed banana. The nine 'fishin' chimps' spent an average of only 2 minutes per day at the mound, and the most interested individual only spent 4.2 minutes per day at the mound, 3 minutes of which involved preparing tools and dipping. However, they were involved in an activity that otherwise they would not show. A similar device with orangutans [153] has proved more successful increasing foraging time to 47% .We modified the device making it less expensive to construct and less time consuming to bait [29] (Figure 5).

Without the opportunity to search for and process food, certain animals continue to perform quite normally—like hamsters piling food; some perform behaviour in inappropriate ways—like raccoons washing food in their water dish or stereotyped pacing; [67] some perform food-related behaviour in detrimental ways—like regurgitation and coprophagy in primates. [79]
It seems that animals use abnormal behaviour to improve their condition. In pigs housed in stalls or kept in tethers, stereotypies can occupy as much as 60% of the day. [14] The ability of stereotyped behaviour to lower adrenocortical levels suggests that stereotypy is stress reducing. In very simple enclosures it may also be impossible to perform certain behaviour at all, like dust bathing in chickens, [53,87] wood gouging for gum in marmosets, [40,108] and indeed foraging in most primates. [131, 152]
There are those who will argue that because a stereotypy is an adaptation to an abnormal condition, that it is somehow beneficial and therefore good. This argument seems to be fallacious, like arguing that the fever following infection is good in itself and not merely beneficial during a brief period of abnormal challenge.
Control
Cages are believed to reduce the degree of control that individuals have. When rooms are visually divided, male macaques cannot control female aggression and aggression can increase. [58,60] This change in behaviour after the imposition of dividers does not happen in stable callitrichid monkey groups [109] or in stable macaque groups. [59]
Reduction of control by being in cages is also exemplified by the effects of visitors on the behaviour of zoo animals. [39,76,86,152] One can view zoo visitors as a stimulus in an otherwise barren environment, and certainly some primates interact with visitors. An alternative to viewing visitors as a beneficial stimulus, supported by an increasing amount of evidence,39 is that visitors change the behaviour of monkeys in a way similar to that caused by other stressors (see Table 1). The data suggest that the greater the number of visitors, the more behaviour is changed; noisy and active visitors cause more change; and visitors located at a level above monkeys cause more change than those at a lower level. This research suggests the importance of refuges for the animals, to let them control access to and by visitors. [1,66,100]
Other evidence for the importance of control is the surprising preference animals have for working for part of their food in the presence of free food (previous references).
There is currently discussion on the importance of control in the psychological health of both animals and humans. It is clear that in the wild, animals have more control over certain stimulus variables and over most response variables in comparison with animals in captivity. Such control is restricted in cages, particularly approach and withdrawal responses with respect to stimuli outside cages, withdrawal responses to stimuli within cages, and the opportunity to produce effective motor responses. The discrepancies between control of stimulus and response variables in the captive versus natural environment may be an important idea underlying enrichment studies.
A study to assess directly the effects of early control over the environmental events on socioemotional development showed that young rhesus monkeys that could work in order to receive food and water, showed less self-directed behaviour and were more exploratory than monkeys receiving the same rewards independent of their behaviour. [111] Thus in order to produce behaviourally competent individuals, one could argue that animals should be allowed to execute motor acts on their environment which have relevant consequences. Extreme loss of control leads to syndromes such as 'learned helplessness'134 and to individuals that use their own body to modulate their emotional states. [5, 112]
Where animals do have a type of control, the opportunity to withdraw (for example, from predators) means that for the wild animal, the period of arousal is usually brief; in captivity this is not the case (for example, in zoos). Even when stressors are prolonged, the wild individual has options. From personal observations, it seems likely that in many cercopithecus species it is stressful for young adult males to remain in their natal group, as evidenced by a slowing of growth and development. When they withdraw or are withdrawn, one can see a marked growth spurt. [25]
Complexity/ predictability
On a more conceptual level, many cage environments can be thought of as reducing the complexity and increasing the predictability of stimulation. In a predator-free or predator-predictable environment (such as, laboratory or zoo) or in an environment where only one type of food is offered, decisions are more simple, alternatives fewer, stimuli more repetitive and predictable.
EVALUATION OF SUCCESS OF ENRICHMENT
Assessment should be seen as crucial in studies of the effects of enrichment, since it answers the questions—Does the manipulation/enrichment do what it is designed to do? Are the effects reliable? And does it do it well enough to be worth the effort, that is, does the benefit exceed the total cost?
The cost is most often viewed in terms of time or money, but can also be measured in terms of alternatives. If one form of enrichment is chosen, then other potential forms may have to be excluded. If a ball or branch is chosen as the enrichment device (for example, [125,128]), then this may be at the expense of other possible devices. The cost in baiting time of the tree stumps shown in Figure 6 was so great that the device will not be used despite having some benefits. One can view the evaluation of an enrichment technique along similar lines to that of a new drug. If it is the first useful drug, then the drug is compared with that of a placebo, unless its benefits are so valuable or so obviously above that of known placebo effects to make such a trial unnecessary (for example, vaccination for smallpox). If it is an improved drug, then it is compared with previous drugs used for that illness.

Just as cost can be measured from several aspects, so can benefit. Both the short- and long-term effects of an intervention need to be measured and criteria for the duration of the effect need to be specified. For example, a small controlled fire in a large enclosure for chimpanzees led to a dramatic change in behaviour for a short period of time during the first presentation; but interest was not maintained after the second session [29] and the fire was not used as a basis for tool use. It is usually long- term changes in behaviour that are of interest and measured in competent studies of enrichment (for example, [149]). Changes in behaviour usually need to occur frequently during the day, and they need to be maintained over long periods of time to be considered effective and worthwhile.
Finally, to measure benefit one needs to decide the importance of behaviour which is altered. Two extremes will illustrate the difficulty in such a decision. Certain behaviour may be considered important because it normally occurs frequently, such as foraging, locomotion, or huddling. King and Norwood [96] consider the opportunity to leap to be important in squirrel monkeys, as over 40% of their travel is accomplished by leaping. Other behaviour which may be considered important precisely because it normally occurs infrequently (see Figures 5 and 6). Dust bathing in fowl is an example [46] or food-offering and sharing in tamarins. [67] And still other behaviour which maybe important, is infrequent behaviour which when used may actually lead to the reduction of more common behaviour. For example allowing the use of tools enables capuchin monkeys to process certain nuts four times as rapidly as without tools [151] while the range of their behaviour increases.
As an example of these principles of assessment, between 1979 and 1986 a series of studies assessed the effects of covering the floor with a deep, absorbent litter in the large cages of eight different primate species. [6,30,34,35,109] The goal was to improve welfare, especially to increase activity (discussed later) and reduce abnormal and undesirable behaviour shown in some of these species.
The technique of enrichment used a 4 cm-deep floor-covering of woodchips with small items of prized food mixed into it. The choice of floor-covering was not one of emulation—to copy nature, as this is impractical for almost all captive environments; rather the aim was simulation—to provide certain, possibly critical, components of the natural environment.
The success of the technique was measured over several months to determine costs and benefits and the stability of the behavioural changes. Technician workload was assessed, cage cleanliness and odour were evaluated, and changes in the animals' behaviour was recorded. Bacterial vigour in the woodchips was also measured to evaluate health implications. Finally a hypothesis was tested—that enrichment would be more effective when targeting behaviour more common to the animal in its natural setting. Therefore it was predicted (1) that changing the opportunity for an important and common behaviour pattern like foraging would lead to substantial changes; and (2) that arboreal species would be less affected by providing a floor-covering than would more terrestrial species, since the former are less likely to spend time foraging at ground level. [85]
The results of these studies clearly showed the high cost-effectiveness of the floor-covering in improving behaviour. The desired goals were achieved in the initial studies on stumptail macaques; self-injurious behaviour was reduced by more than half; [5] aggression was reduced by a factor of 2 in adults and in juveniles by a factor of 10. Similar reductions occurred in all but one of the eight species subsequently studied and in which aggression was seen.
When the floor of the cage or enclosure was bare, not surprisingly macaques spent almost no time searching for food items on it. When the floor was covered with woodchips only, the animals spent about 5% of their waking time searching through this floor-covering. When grain was added to the woodchips, the monkeys' searching increased to almost 15% of their waking time, even when grain was also freely available elsewhere. When free grain was removed so that the only grain available had to be found and extracted from the woodchips, the monkeys spent over 30% of their day foraging. This percentage more closely resembles the behaviour of free-ranging macaques. [32,33]
All the eight primate species assessed have shown increased use of the floor area when it was covered with woodchips. We were surprised to find no support for our prediction that arboreal primates would benefit less from the floor-covering: all groups showed increases in floor use. The increased time spent foraging was at the expense of other behaviour: Aggression and abnormal behaviour were reduced, as was the amount of time spent inactive, and so also were play and affiliative behaviour but to a lesser extent.
The cost of this procedure was assessed in several ways. The time required for cleaning was reduced from 5 to 2 hours per week per enclosure when woodchips were used. The enclosures, especially the walls and windows, were judged to be cleaner, and they were rated as smelling less after four weeks with woodchips than after one day with a bare floor cleaned with water, detergent, and disinfectant. To assess the potential for the spread of disease when using the litter, samples of woodchips were removed periodically over eight weeks and either (a) tested for the presence of bacteria, or (b) inoculated with Salmonella bacteria and its survival time measured. Similar to tests using chickens, [143] the results showed that the longer the litter was in use, the more inhibitory it was to bacterial survival.
Two studies illustrate the complexity of proper evaluation of enrichment procedures. Rosenblum and Smiley [131] found that providing a foraging task reduced behaviour such as self-aggression and abnormal posturing in high- and low-ranking members of a group of isolation-reared bonnet macaques, but these behaviour patterns increased in intermediate-ranking monkeys. Bloomstrand et al. [16] reported behavioural deterioration in certain members of a chimpanzee group with access to a food puzzle, while other members' improved (also [6, 20,104]). It. seems likely that such negative reactions arise from social tensions created by the competitive nature of these two enrichment tasks. In the wild it is uncommon for animals to be competing for food massed into a small area. Consequently, it might be suggested that either such enrichment devices are particularly suited to individually-housed animals, or that several devices be offered to the group simultaneously.
Although concentrating on cage size and design in this review, these are not the only problems to be confronted by those desiring enrichment. Providing social interaction for a social species seems very important to social humans. There are many studies which have looked at this problem in pair- and individually-housed monkeys, and in infants and adults. [24,27,35,36,37.67,77, 102, 124] The abnormal behaviour patterns caused by social deprivation or restriction are no less dramatic or salient, [5] and social primates seem strongly motivated to maintain physical social contact (Figure 7).

Acknowledgements
The following made helpful comments on the text or in discussion: J. R. Anderson, H. 0. Box, R. Cooper, S. Evans, A. T. C. Feistner, W. C. McGrew, E. Moodie, E. Price, C. T. Snowdon, F. Wemelsfelder.
References
1. Adams, S. and Babladelis, G. (1977). An ecological approach to animal groups in zoos. International Zoo News, 24: 14-22.
2. Ainslie, G. (1975). Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin, 82: 463-496.
3. Akers, J. S. and Schildkraut, D. S. (1985). Regurgitation/reingestion and coprophagy in captive gorillas. Zoo Biology, 4: 99-109.
4. Albright, J. L. (1982). Production changes improve veal cow welfare. Feedstuffs, 12: 23-33.
5. Anderson, J. R. and Chamove, A. S. (1981). Self-aggressive behaviour in monkeys. Current Psychological Reviews, 1: 139-158.
6. Anderson, J. R. and Chamove, A. S. (1984). Allowing captive primates to forage. In Standards in Laboratory Animal Management. Symposium Proceedings (Vol. 2). Potters Bar, England, Universities Federation for Animal Welfare.
7. Anderson, J. R. and Chamove, A. S. (1984). Early social experience and responses to visual social stimuli in young monkeys. Current Psychological Research and Reviews, 3: 32-45.
8. Archer, J. (1979). Animals Under Stress. London, Edward Arnold.
9. Bareham, J. R. (1972). Effects of cages and semi-intensive deep litter pens on the behaviour, adrenal response and production in two strains of laying hens. British Veterinary Journal, 128: 153-163.
10. Belzung, C. and Anderson, J. R. (1986). Social rank and responses to feeding competition in rhesus monkeys. Behavioural Processes, 12: 307-316.
11. Berkson, G. (1974). Social responses of animals to infants with defects. In Effects of the Infant on its Caregiver. Chevalier-Skolnikoff, S. and Poirier, F. E. (eds). London, Wiley.
12. Berkson, G., Mason, W. and Saxon, S. (1963). Situation and stimulus effects on stereotyped behaviors of chimpanzees. Journal of Comparative and Physiological Psychology, 56: 786-792.
13. Beuving, G. (1980). Corticosteroids in laying hens. In The Laying Hen and its Environment. Moss, R. (ed). The Hague, Martinus Nijhoff.
14. Blackshaw, J. K. and McVeigh, J. F. (1984). Stereotype behavior in sows and gilts housed in stalls, tethers, and groups. In Advances in Animal Welfare Science, 1984. Fox, M. W. and Mickley, L. D. (eds). Boston, Martinus Nijhoff Publishers.
15. Bloomstrand, M., Alford, P. L. and Maple, T. L. (1987). An analysis of feeding enrichment for captive chimpanzees. Paper presented at meeting American Society of Primatalogists, Madison, WI.
16. Bloomstrand, M., Riddle, K., Alford, P. and Maple, T. L. (1986). Objective evaluation of a behavioral enrichment device for captive chimpanzees (Pan troglodytes). Zoo Biology, 5: 293-300.
17. Boccia, M. L. ( 1989) .Preliminary report on the use of a natural foraging task to reduce aggression and stereotypies in socially housed pigtail macaques. Laboratory Primate Newsletter, 28: 3-4.
18. Bollhom, R. W. (1980). Lighting in the animal environment. Laboratory Animal Science, 30: 440-451.
19. Bovard, R. (1959). Social stimulation and the response to stress. Psychological Reviews, 58: 267-287.
20. Brambell, M. R. (1977). Reintroduction. International Zoo Yearbook, 17: 112-116.
21. Bryant, C. E., Rupniak, N. M. J. and Iversen, S. D. (1988). Effects of different environmental enrichment devices on cage stereotypies and autoaggression in captive cynomolgus monkeys.Journal of Medical Primatology, 17: 257-267.
22. Carder, B. and Berkowitz, K. (1979). Rats' preference for earned in comparison with free food. Science, N.Y., 167: 1273-1274.
23. Chamove, A. S. (1989). Cage design reduces emotionality in mice. Laboratory Animals, 25: 215-219.
24. Chamove, A. S. (1980). Nongenetic induction of acquired levels of aggression. Journal of Abnormal Psychology, 89: 469-488.
25. Chamove, A. S. (1981). Establishment of a breeding colony of stumptailed monkeys. Laboratory Animal, 15: 251-259.
26. Chamove, A. S. (1984). Long-term learning deficits of mentally retarded monkeys. American Journal of Mental Deficiency, 88: 352-368.
27. Chamove, A. S. (1984). Role of vision in social interaction in monkeys. Child Development, 55: 1394-1411.
28. Chamove, A. S. (1986). Exercise improves behaviour: A rationale for occupational therapy. British Journal of Occupational Therapy, 49: 83-86.
29. Chamove, A. S. (in press). Enrichment in chimpanzees: Fire, unpredictable ropes, and tools. Ratel.
30. Chamove, A. S. and Anderson, J. R. (1979). Woodchip litter in macaque groups. Journal of the Institute of Animal Technicians, 30: 69-74.
31. Chamove, A. S. and Anderson, J. R. (1981). Self-aggression, stereotypy, and self- injurious behaviour in man and monkeys. Current Psychological Reviews, 1: 245-256.
32. Chamove, A. S. and Anderson, J. R. (1988). Impact of feeding practices on growth and behavior of stumptailed macaques. The Ecology and Behavior of Food-Enhanced Primate Groups. In Fa, I. E. and Southwick, C. H. (eds). New York, A. R. Liss Inc.
33. Chamove, A. S. and Anderson, J. R. (1989). Examining environmental enrichment. The Psychological Well-Being of Primates. In E. Segal (ed). Philadelphia, Noyes Pub. Co.
34. Chamove, A. S., Anderson, J. R., Morgan-Jones, S. C. and Jones, S. P. (1982). Deep woodchip litter: Hygiene, feeding, and behavioural enhancement in eight primate species. International Journal for the Study of Animal Problems, 3: 308-318.
35. Chamove, A. S., Anderson, J. R. and Nash, V. J. (1984). Social and environmental influences on self-aggression in monkeys. Primates, 25: 319-325.
36. Chamove, A. S., Bayart, F., Nash, V. J. and Anderson, J. R. (1985). Dominance, physiology, and self-aggression in monkeys. Aggressive Behaviour. 11: 17-26.
37. Chamove, A. S. and Bowman, R. E. (1978). Rhesus plasma cortisol response at four dominance positions. Aggressive Behaviour, 4: 43-55.
38. Chamove, A. S. Callitrichid monkeys prefer many, unevenly-spaced branches (in preparation).
39. Chamove, A. S., Hosey, J. and Schaetzel, P. (1988). Visitors excite primates in zoos. Zoo Biology, 7: 359-369.
40. Chamove, A. S. and Rohrhuber, B. (1989). Moving callitrichid monkeys from cages to outside areas. Zoo Biology. 8: 151-163.
41. Chapman, C. (1985). The influence of habitat on behaviour in a group of St. Kitts green monkeys. Journal of Zoology, London (A). 206: 311-320.
42. Cherfas, J. (1984). Zoo 2000. London, British Broadcasting Corporation.
43. Clark, M. M. (1980). Effects of rearing environment on adrenal weights, sexual development, and behavior in gerbils: An examination of Richter's domestication hypothesis. Journal of Comparative and Physiological Psychology. 94: 857-863.
44. Clutton-Brock, T. H. and Harvey, P. H. (1977). Species differences in feeding and ranging behaviour in primates. In Primate Ecology. Clutton-Brock, T. H. (ed). London, Academic Press.
45. Dantzer, R. and Mormede, P. (1983). Stress in farm animals: A need for re-evaluation. Journal of Animal Science, 57: 6-18.
46. Dawkins, M. (1976). Towards an objective method of assessing welfare in domestic fowl. Applied Animal Ethology, 2: 245-254.
47. Dawkins, M. (1977). Behavioral wisdom lost or hidden? Applied Animal Ethology, 3: 194-195. 48. Dawkins, M. S. (1980). Animal Suffering, the Science of Animal Welfare. London, Chapman arid Hall.
49. Denenberg, V. H. (1964). Critical periods, stimulus input, and emotional reactivity: A theory of infantile stimulation. Psychological Review, 71: 335-351.
50. Denenberg, V. H. and Whimbbey, A. E. (1963). Behavior of adult rats is modified by the experiences their mothers had as infants. Science, 142: 1192-1193.
51. Doolittle, D. P., Wilson, S. P. and Geisking, D. (1976). Effect of caging variables on bodyweight and weight gain in mice. Laboratory Animal Science, 26: 556-559.
52. Draper, W. A. and Bernstein, I. S. (1963). Stereotyped behavior and cage size. Perceptual and Motor Skills, 16: 231.
53. Duncan, I. J. H. (1977). Behavioral wisdom lost? Applied Animal Ethology, 3: 193-194.
54. Duncan, I. J. H. (1978). The interpretation of preference tests in animal behavior. Applied Animal Ethology, 4: 197-200.
55. Ekesbo, I. (1981). Some aspects of sow health and housing. In The Welfare of Pigs. Sybesma, W. (ed). The Hague, Martinus Nijhoff.
56. Elton, R. H. (1979). Baboon behavior under crowded conditions. pp. 125-138. In Erwin, J., Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
57. Erwin, J. (1979). Aggression in captive macaques: Interaction of social and spatial factors. pp. 139-171. In Erwin,J., Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
58. Erwin, J. (1971). Factors influencing survival and development of Macaca nemestrina and Macaca fascicularis infants in a harem breeding situation. In Cognitive Processes of Nonhuman Primates. Jarrard, L. E. (ed). New York, Academic Press.
59. Erwin, J., Anderson, B., Erwin, N., Lewis, L. and F1ynn, D. (1976). Aggression in captive groups of pigtail monkeys: Effects of provision of cover. Perceptual Motor Skills, 42: 219-224
60. Erwin, J. and Deni, R. (1979). Strangers in a strange land: Abnormal behaviors or abnormal environments? pp. 1-28. In Erwin, J., Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
61. Eisen, E. J. ( 1966) .Comparison of two cage-rearing regimes on reproductive performance and bodyweight of the laboratory mouse. Laboratory Animal Care, 16: 447-453.
62. Evans, S. (1984). Captive management of marmosets and tamarins. In Standards in Laboratory Animal Management. Symposium Proceedings (Vol. 2). Potters Bar, England, Universities Federation for Animal Welfare.
63. Ewbank, R. (1973). Use and abuse of the term 'Stress' in husbandry and welfare. Veterinary Records, 30: 709-710.
64. Feistner, A. T. C. and Chamove, A. S. (1986). High motivation toward food increases food-sharing in cotton-top tamarins. Developmental Psychobiology, 19: 439-452.
65. Folkins, C. H. and Sime, W. E. (1981). Physical fitness training and mental health. American Psychologist, 36: 373-389.
66. Fouts, R. S., Abshire, M. L., Bodamer, M. and Fouts, D. H. (1989). Signs of enrichment: Toward the psychological well-being of chimpanzees. The Psychological Well-Being of Primates. In Segal. E. (ed). Philadelphia, Noyes Pub. Co.
67. Fox, M. W. (1986). Laboratory Animal Husbandry: Ethology, Welfare and Experimental Variables. New York, State University of New York Press.
68. Fraiberg, S. (1974). Blind infants and their mothers. In Effects of the Infant on its Caregiver. Chevalier-Skolnikoff, S. and Poirier, F. E. (eds). London, Wiley.
69. Fraser, A. F. (1980). Ethology, welfare and preventive medicine for livestock. Applied Animal Ethology, 6: 103-109.
70. Garber, P. A. (1980). Locomotor behaviour and feeding ecology of the Panamanian tamarin (Saguinus oedipus geoffroyi, Callitrichidae Primates). International Journal of Primatology, 1: 185-201.
71. Garber, P. A. (1986). Influence of group size on dietary and foraging patterns in Saguinus mystax and Saguinus fuscicollis in Amazonian Peru. Primate Report, 14: 12.
72. Garber, P. A. and Sussman, R. W. (1984). Ecological distinctions between sympatric species of Saguinus and Sciurus. American Journal ofPhysical Anthropology, 65: 135-146.
73. Garmezy, N. (1982). Children under stress. In Further Exploration in Personality. Zucker, R. A. and Rabin, A. I. (ed). New York, Wiley.
74. Gergen, K. J., Gergen, M. M. and Barton, W. H. (1973). Deviance in the dark. Psychology Today, 7: 129-131.
75. Gilbert, S. G. and Wrenshall, E. (1989). Environmental enrichment for monkeys used in behavioral toxicology studies. The Psychological Well-Being of Primates. In Segal, E. (ed). Philadelphia, Noyes Pub Co.
76. Glatston, A. R., Geilvoet-Soeteman, E., Hora-Pecek, E. and van Hooff, J. A. R. A. M. ( 1984) .The influence of the zoo environment on social behavior of groups of cotton-topped tamarins,Saguinus oedipus oedipus. Zoo Biology, 3: 241-253.
77. Goosen, C. (1980). On Grooming in Old World Monkeys. Meinema, W. D., Delft. 78. Goosen, C., Fransen, S. and Gommers, M. V. D. (1986). Social aspects of abnormal locomotion stereotypy. Primate Report, 14: 166.
79. Gould, E. and Bres, M. (1986). Regurgitation and reingestion in captive gorillas: Description and intervention. Zoo Biology, 5: 241-250.
80. Gross, W. (1972). Effect of social stress on occurrence of Marek's disease in chickens. American Journal of Veterinary Research, 33: 2275-2279.
81. Harlow, H. F. (1950). Learning and satiation of responses in intrinsically motivated complex puzzle performance by monkeys. Journal of Comparative and Physiological Psychology, 43: 289-294.
82. Harlow, H. F. (1971). Learning to Love. San Francisco, Albion.
83. Harrison, M. J. S. (1983). Age and sex differences in the diet and feeding strategies of the green monkey, Cercopithecus sabaeus. Animal Behaviour 969-977.
84. Havelka, J. (1956). Problem-seeking behaviour in rats. Canadian Journal of Psychology, 10: 91-97.
85. Heyman, E. W. ( 1987) .A field observation of predation on a moustached tamarin Saguinus mystax by an anaconda. International Journal of Primatology, 8: 193-194.
86. Hosey, G. R. and Druck, P. L. (1987). The influence of zoo visitors on the behaviour of captive primates. Applied Animal Behaviour Science, 18: 19-29.
87. Hughes, B. 0. (1976). Preference decisions of the domestic hen for wire or litter floors. Applied Animal Etholgy, 2: 155-165.
88. Hughes, B. 0. (1977). Behavioural wisdom and preference tests. Applied Animal Ethology, 3: 391-392.
89. Hughes, B. 0. (1980). The assessment of behavioural needs. In The Laying Hen and its Environment. Sybesma, V. (ed). The Hague, Martinus Nijhoff.
90. Imamura, S. (1965). Mother and Blind Child. New York, American Foundation for the Blind.
91. Inglis, I. R. and Ferguson, N. J. K. (1985). Starlings search for food rather than eat freely-available identical food. Animal Behaviour, 34: 614-616.
92. Kalin, N. H., Carnes, M., Barksdale, C. M., Shelton, S. E., Stewart, R. D. and Risch, S. C. (1985). Effects of acute behavioural stress on plasma and cerebrospinal fluid ACTH and B-endorphin in rhesus monkeys. Neuroendocrinology, 40: 97-101.
93. Kaplan, J. R. (1986). Psychological stress and behavior in nonhuman primates. In Mitchell, G. and Erwin, J.(eds). Comparative Primate Biology, vol. 2, part A: Behaviour, Conservation, and Ecology. New York, Alan R. Liss.
94. Kear, J. (1961). Food selection in finches with special reference to interspecific differences. Proceedings of the Zoological Society of London, 138: 163-203.
95. Kendrick, D. C. (1972). The effects of stimulation and intermittent fasting and feeding on life span in the black-hooded rat. Development Psychobiology, 6: 225-234.
96. King, J. and Norwood (1989). Free environment rooms as alternative housing for squirrel monkeys. The Psychological Well-Being of Primates. In Segal, E. (ed). Philadelphia, Noyes Pub. Co.
97. Kinzey, W. G. (1986). Feeding, travel distance and group size in Callicebus torquatus. Primate Report, 14: 11.
98. Kirkwood, J. K. and Dow, S. M. (1986). Feeding primates in captivity, nutritional and behavioural considerations. Primate Report, 14: 42.
99. Koomans, P. (1981). Open front piggeries with and without straw. In The Welfare of Pigs. Sybesma, W. (ed). The Hague, Martinus Nijhoff.
100. Maple, T. L. (1979). Great apes in captivity: The good, the bad, and the ugly. pp. 239-272. In Erwin, J. Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
101. Marriott, B. (1986). Social influence on activity-based energy intake and expenditure in free-ranging rhesus monkeys. Primate Report, 14: 151.
102. MacKenzie, M. M., McGrew, W. C. and Chamove, A. S. (1985). Social preferences in stumptailed macaques (Macaca arctoides): Effects of companionship, kinship, and rearing.Development Psychobiology, 18: 115-123.
103. Markowitz, H. (1979). Environmental enrichment and behavioral engineering for captive primates. pp. 217-238. In Erwin, J., Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
104. Markowitz, H. (1982). Behavioral Enrichment in the Zoo. New York, Van Nostrand Reinhold.
105. Markowitz, H. and Spinelli, J. S. (1986). Environmental engineering for primates. In Benirschke, K. (ed). Primates: The Road to Self-Sustaining Populations. New York, Springer-Verlag.
106. McFarland, M. J. (1986). Food competition and foraging group size in the black spider monkey, Ateles paniscus. Primate Report, 14: 12.
107. McGrew, W. C. (1981). Social and cognitive capabilities of nonhuman primates; Lessons from wild to captivity. International Journal for the Study of Animal Problems, 2: 138-149.
108. McGrew, W. C., Brennan, J. A. and Russell, J. (1986). An artificial 'gum tree' for marmosets (Callithrix j. jacchus). Zoo Biology, 5: 45-50.
109. McKenzie, S. M., Chamove, A. S. and Feistner, A. T. C. (1986). Floorcoverings and hanging screens alter arboreal monkey behavior. Zoo Biology, 5: 27-39.
110. Millar, S. K., Evans, S. and Chamove, A. S. (1988). Oldest offspring contact novel soonest in callitrichid families. Behavioural Biology, 13: 82-96.
111. Mineka, S., Gunnar , M. and Champoux, M. (1986). Control and early socioemotional development: Infant rhesus monkeys reared in controllable versus uncontrollable environments. Child Development, 57: 1241-1256.
112. Mitchell, G., Maple, T. L. and Erwin, J. (1979). Development of social attachment potential in captive rhesus monkeys. pp. 59-124. In Erwin, J., Maple, T. L., Mitchell, G. (eds). Captivity and Behavior of Primates in Breeding Colonies, Laboratories and Zoos. New York, Van Nostrand Reinhold.
113. Moodie, E. M. and Chamove, A. S. Brief excitement beneficial for captive tamarins? Zoo Biology (in press).
114. Nash, V. J. (1982). Tool use by captive chimpanzees at an artificial termite mound. Zoo Biology, 1: 211-221.
115. Neuringer, A. J. (1970). Animals respond for food reward with free food present. Sciences, N.Y., 166: 399-401.
116. Neyman, P. F. (1980). Ecology and social organization of the cotton-top tamarin. PhD. dissertation, University of California, Berkeley.
117. Oates, J. F. (1986). Food distribution and foraging behavior. In Primate Societies. Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., Struhsaker, T. T. (eds). Chicago, University of Chicago Press.
118. O'Neill, P. (1989). A room with a view for captive primates: Issues, goals, related research and strategies. The Psychological Well-Being of Primates. In Segal, E. (ed). Philadelphia, Noyes Pub. Co.
119. Parker, S. T. and Gibson, K. R. ( 1979) .A developmental model for the evolution of language and intelligence in early hominids. The Behavioural and Brain Sciences, 2: 367-408.
120. Paulk, R. R., Dienske, R. and Ribbens, L. G. (1977). Abnormal behavior in relation to cage size in rhesus monkeys. Journal of Abnormal Psychology, 86: 87-92.
121. Putten, G. van and Dammers, J. (1976). A comparative study of the well-being of piglets reared conventionally and in cages. Applied Animal Ethology, 2: 339-356.
122. Regal, D. M., Both, R., Teller, D. Y. and Sackett, G. P. (1987). Visual acuity and visual responsiveness in dark-reared monkeys (Macaca nemestrina). Vision Research, 16: 532-530.
123. Reinhardt, V. (1987). Improved installation method for branches as cage enrichment. Laboratory Primate Newsletter, 26: 1.
124. Reinhardt, V., Cowley, D., Eisele, S., Vertein, R. and Houser, D. (1987). Preliminary comments on pairing unfamiliar adult female rhesus monkeys for the purpose of environmental enrichment. Laboratory Primate Newsletter, 26: 5-8.
125. Reinhardt, V., Houser, W. D., Cowley, D. and Champoux, M. (1987). Preliminary comments on environmental enrichment with branches for individually caged rhesus monkeys. Laboratory Primate Newsletter, 26: 1-3.
126. Reinhardt, V., Reinhardt, A. and Houser, W. D. (1986). Partner directed hair-pulling and -eating in (Macaca mulatta). Primate Report, 14: 166.
127. Renner, M. J. and Rosenzweig, M. R. (1987). Enriched and Impoverished Environments. London, Springer-Verlag.
128. Renquist, D. M. and Judge, F. J. (1984). Use of nylon balls as behavioral modifier for caged primates. Laboratory Primate Newsletter, 24: 4.
129. Richard, A. F. (1985). Primates in Nature. New York, W. H. Freeman & Co.
130. Riley, V. (1981). Psychoneuroendocrine influence on immunocompetence and neoplasia. Science, 212: 465-467.
131. Rosenblum, L. A. and Smiley, J. (1984). Therapeutic effects of an imposed foraging task in disturbed monkeys. Journal of Child Psychology and Psychiatry, 25: 485-497.
132. Sackett, G. P. (1966). Monkeys reared in isolation with pictures as visual input. Evidence for an innate releasing mechanism. Science, 154: 1468-1470.
133. Sackett, G. P. (1970). Innate mechanisms, rearing conditions, and a theory of early experience effects in primates. In Miami Symposium on the prediction of behavior: Early Experience. Jones, R. M. (ed). Miami, University of Miami Press.
137. Seligman, M. E. P. (1975). Helplessness: On Depression, Development and Death. San Francisco, W. H. Freeman.
135. von Senden, M. (1960). Space and Sight. London, Methuen.
136. Siegel, R. S. (1980). Physiological stress in birds. Bioscience, 30: 529-534.
137. Singh, S. D. (1969). Urban monkeys. Scientific American, 221: 108-115.
138. Snowdon, C. T. (1989). The criteria for successful captive propagation of endangered primates. Zoo Biology Supplement, 1: 149-161.
139. Snowdon, C. T., Savage, A. and McConnell, P.B. (1984). A breeding colony of cotton- top tamarins (Saguinus oedipus). Laboratory Animal Science, 35: 4-77-480.
140. Stolba, A. and Wood-Gush, D. G. M. (1980). Arousal and exploration in growing pigs in different environments. Applied Animal Ethology, 6: 382-383.
141. Sussman, R. W. and Kinzey, W. G. (1984). The ecological role of the Callitrichidae: A review. American Journal of Physical Anthropology, 64: 419-449.
142. Tripp, J. K. (1985). Increasing activity in captive orangutans: Provision of manipulable and edible materials. Zoo Biology, 4: 225-234.
143. Turnbull, P. C. B. and Snoyenbos, G. H. (1973). The roles of ammonia, water activity, and pH in the salmonellacidal effect of long-used poultry litter. Poultry Science, 12: 72-86.
144. Turnquist, J. E. (1986). Gang-caged versus free-ranging rhesus monkeys: A comparison of body proportions and passive joint mobility. Primate Report, 14: 171.
145. Unshelm, J. Losses due to inheritance and housing as criteria for the welfare of pigs. In The Welfare of Pigs. Sybesma, W. (ed). The Hague, Martinus Nijhoff.
146. Vestergaard, K. (1981). Influence of fixation on the behaviour of sows. In The Welfare of Pigs. Sybesma, W. (ed). The Hague, Martinus Nijhoff.
147. Waser, P. (1986). Interactions among primate species. In Primate Societies. Smuts, B. B., Cheney, D. L., Seyfarth, R. M.,Wrangham, R. W., Struhsaker, T. T. (eds). Chicago, University of Chicago Press.
148. Wemelsfelder, F. (1985). Animal boredom: Is a scientific study of the subjective experiences of animals possible? In Advances in Animal Welfare Science, 1984. Fox, M. W., Mickley, L. D. (eds). Boston, Martinus Nijhoff Publishers.
149. Westergaard, G. C. and Fragazy, D. M. (1985). Effects of manipulatable objects on the activity of captive capuchin monkeys (Cebus apella). Zoo Biology, 4: 317-327.
150. Wood-Gush, D. G. M. (1973). Animal welfare in modem agriculture. British Veterinary Journal, 129: 164-174.
151. Visalberghi, E. and Antinucci, F. (1986). Tool use in the exploitation of food resources in Cebus apella. In Primate Ecology and Conservation. Vol. 2. Else, J. C., Lee, P. C. (eds). London, Cambridge University Press.
152. Worsley-White, C. J. (1988). Effect of zoo visitors on the social behaviour, activity , and audience-awareness of three species of captive primate. Bolton Institute of Higher Education, unpublished manuscript.
153. McEwan, P. (1986). Environmental enrichment: An artificial termite mound for orangutans. Potters Bar, Herts, UFAW.
154. Bantin, G. C. and Sanders, P. D. (1989). Animal caging: Is big necessarily better? Animal Technology, 40: 45-54.
155. Finlay, T ., James, L. R. and Maple, T. L. (1988). People's perceptions of animals: The influence of the zoo environment. Environment and Behavior, 20: 508-528.
Reproduced with permission of the Institute of Animal Technology. Published in: Animal Technology (1989) Vol. 40, No.3