Amy Kessel and Linda Brent
Department of Laboratory Animal Medicine,
Southwest Foundation for Biomedical Research,
San Antonio, Texas 78228-0147
SUMMARY
The most beneficial way to enrich the life of an individually housed primate has been demonstrated to be that of providing a social companion. Where this is not an option, inanimate enrichment options have been employed. Enrichment methods for individually housed primates commonly consist of providing toys, structures, different types of food, or feeding puzzles to the animals. In an attempt to provide individually housed chimpanzees with a source of animate, and possibly interactive, enrichment, seven chimpanzees (Pan troglodytes) were provided with a single goldfish in a small plastic aquarium. The subjects were unable to touch the fish, but could touch the aquarium and its Plexiglas holder. Subjects were observed for 8 weeks, with fish being provided in one-week intervals randomly distributed throughout the time period. Data were collected with paper and pen using a 15-minute scan sampling method. Observations were balanced for time of day and condition. Data that were summarized and used for analysis totaled 112 hours. The chimpanzees spent 3.53 percent of the data points engaged in fish directed behavior. Analysis of the data by behavior category did not result in any significant differences related to the provision of the fish. Fish directed behavior occurred significantly more in the first week of exposure than in the following 3 weeks (F = 51.67, p <0.001) and rapidly declined thereafter.
INTRODUCTION
Housing captive nonhuman primates in single cages has been shown to have detrimental effects on their behavior.1,2 Although current regulations in the United States require that singly housed primates must be able to see and hear others of their species, other improvements can be made.3 Some successful improvements have been pairing animals or moving them into social groups,4,5 or even providing access to conspecifics on an irregular basis, such as with the use of a playroom or activity cage.6,7,8,9 In some cases, however, social enrichment is not possible due to research protocols or management concerns, such as compatibility issues.
When social enrichment is not an option, we may attempt enrichment techniques that give the animal more control over its environment. Control is an aspect of the wild environment that is often missing in the captive animal's life. Decisions are made by the human caretakers—from what the animals will eat to when they will eat it. The value of giving laboratory animals control oyer their own environment is currently being explored with radios, televisions, computer games, and some forms of training.10,11,12,13,14 It is hoped that giving nonhuman primates enrichments that can be affected more directly by their actions, will give them greater levels of control and will provide greater benefits than traditional enrichment options.
Traditional enrichment for singly housed nonhuman primates has usually consisted of inanimate objects. These objects have included a variety of toys, structures such as perches, different foods, and feeding puzzles.15 These inanimate enrichers do not respond to the subject's actions nor do they initiate actions on their own.
For nonhuman primates that must be housed singly, enrichment becomes even more important. The use of animate enrichment with singly caged nonhuman primates has not been fully explored. Animate enrichment may be in the form of another species. Nonhuman primates in the wild and in captivity have been known to interact positively with other species on their own initiative. There have been anecdotal stories of nonhuman primates "adopting" other species. One baboon adopted a herd of goats16 and baboons at Southwest Foundation in San Antonio, Texas, have been reported to adopt feral cats.17
Individuals have attempted to enrich captive primates' environment with species such as dogs, cats, and even humans. Canines have been used successfully as surrogates for both nursery-reared macaques and chimpanzees.16,18,19,20 Koko the gorilla has had two kittens as companions during her life, but the cats have not been qualitatively examined as an enrichment option.21 Bayne et al22 concluded that the provision of human interaction for as little as two minutes/day was beneficial to rhesus macaques. Bloomsmith etal23 concluded that positive reinforcement training, which allows the animals to interact with a human trainer and to decide if they will cooperate or not, can be a form of environmental enrichment for chimpanzees.
Fish have also been used as an enrichment option, although more as feeding enrichment than as social enrichment. In a study by King & Norwood,24 a social group of squirrel monkeys was provided with goldfish in either a ceramic crock or wading pools. The monkeys were allowed to capture and consume the goldfish after being accustomed to the flavor of fish. The authors state that the goldfish "elicited a frenzy of excited attempts to grab them" the first time the squirrel monkeys were allowed access to them. Another study describes the capture and consumption of fish by wild chacma baboons.25 Humans use fish as a food source, but humans also seem to enjoy viewing fish in an aquarium. The purpose of this study was to provide goldfish in aquariums to individually housed chimpanzees. We hoped that this passive social enrichment option would provide an animate enrichment that would move about on its own as well as in reaction to the chimpanzees, thereby offering a more novel and possibly more stimulating enrichment than a traditional object or device.
METHODS AND MATERIALS
Subjects
Subjects were two female and five male, individually housed chimpanzees (Pan troglodytes) involved in various biomedical research projects. Individual housing was necessary due to research constraints, although all of the chimpanzees had auditory , visual, olfactory, and sometimes tactile contact with other chimpanzees. The chimpanzees ranged from 7 to 23 years in age (mean = 16.5). The chimpanzees were housed in 4.6 m2 cages with toys, perches, grain feeders, and televisions as standard enrichment. In addition, the chimpanzees were provided with daily enrichment in the form of food and frozen treats, interaction with the enrichment technician, or access to items such as magazines and crayons.
Materials
Small plastic aquariums (Aqua-tarium by Super Pet, Pets International, Ltd., Arlington Hts., Illinois) and goldfish were purchased from a local pet store. The goldfish were kept in a social group in a 20 gallon aquarium when not being used for the study.
Before the study began, a sheet of Plexiglas that served as the aquarium holder was attached to the outside of the chimpanzee's cage with wire. This aquarium holder was left on the cage throughout the study.
During the experimental phase of the study, one goldfish was placed in a small aquarium filled with dechlorinated tap water. Small black aquarium rocks were placed on the bottom of the aquarium. On Monday morning during the experimental phase of the study, the aquarium, containing a single goldfish, was attached to the Plexiglas holder. The aquarium was held in place by wire. The Plexiglas allowed the chimpanzees to see the fish, touch the aquarium and holder, but not the fish itself. Fish were fed daily and were removed each week on Friday afternoon.
Data Collection
Observations were conducted over an 8 week time period and the fish were provided randomly for four one-week blocks. Only the Plexiglas holder was present during the no-fish condition which occurred during the remaining four one-week blocks.
Table 1
| Fish directed behaviours |
|---|
| Attempt to touch |
| Bang aquarium |
| Lick aquarium |
| Look at fish |
| Manipulate aquarium |
| Other |
| Smell fish/aquarium |
Table 2
| Behavior category | Examples of behaviors included |
|---|---|
| Abnormal | Arm circle, feces painting, poke self, regurgitate, rock |
| Affiliative | Groom, reach out to, wrist present |
| Aggressive | Display, spit, sway, tantrum, throw feces |
| Attention-getting | Approach, beg, spit, sway, throw object |
| Apprehension/excitement | Bark, piloerection, scream, whimper |
| Exploration/environment | Carry, lick, manipulate, smell, touch object |
| Feeding | Drink, eat, food grunts |
| Locomotion | Climb, jump, run, walk, swing |
| Play | Acrobatics, laugh, quiet play, object play |
| Rest | Crouch, hang, lie, sit, sleep, stand |
| Self-directed | Groom, lick, manipulate, scratch, smell |
| Sexual | Inspect, masturbate, penis display, present |
| Submissive | Avoid, present |
| Other | Defecate, sneeze, urinate, watch, yawn |
Data were collected with paper and pen using a 15-minute scan sampling method at 15-second intervals. All observations were balanced for condition and time of day. Eight trials were conducted each week on each subject for a total of 112 hours of data collected. Behavioral categories are listed in Table 2. Behaviors could occur in more than one category depending on their context.
Table 1 lists the fish directed behaviors that were recorded. Contact with the Plexiglas was considered as contact with the aquarium in both phases of the study.
Data Analysis
Data were summarized and totals were entered into Systat for Windows Statistical Program (Evanston, Illinois). A repeated measures analysis of variance was used to analyze the data for each behavior category using the presence of fish as a grouping variable. The categories of affiliative, apprehension, submissive, and sexual behaviors were not included in the analysis due to limited occurrence. Fish directed behaviors were compared across the successive weeks of exposure. Significance was defined as p < 0.05 for all categories analyzed.
RESULTS
The chimpanzees spent 3.53% of the data points engaged in fish directed behavior. Activities included looking at the fish for 64.8% of the data points, as well as manipulating the aquarium and attempting to touch the fish (See Figure 1). No significant differences were found related to the provision of the goldfish when the data were analyzed by behavior category (all p > 0.05).
The chimpanzees did interact with the fish significantly more in the first week compared to the following 3 weeks (F = 51.67, p < 0.001) (See Figure 2). The interaction with the fish declined rapidly after the first day of exposure from 7.5% of the observation time in the first week to 1.5% in the 3rd week. The apparent increase in fish-use in the 4th week, although not significant, could be attributed to one young female subject.
The two female subjects interacted with the goldfish more often than did the male subjects (female mean = 25,6%; male mean = 9.8%), although due to the small number of subjects, this could not be analyzed statistically.

FIGURE 1. Percentage of observation time spent in fish directed behaviors.
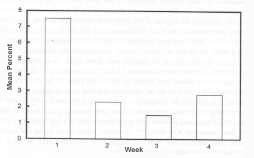
FIGURE 2. Percentage of time spent interacting with the fish over a 4 week time period.
DISCUSSION
Information regarding enrichment options is beneficial to those attempting to improve the well-being of the animals in their care. It is important to report both positive and negative results as well as discussing possible reasons for the results. Negative results may help to avoid repetition of enrichment options that seem to have questionable value, while giving others ideas on ways to improve on a particular enrichment option or perhaps inspiration to develop new and original enrichment options. The reporting of negative results may also help to avoid wasting the time and money necessary to implement an enrichment option that has little effect on the animal's behavior. Contrary to our hopes that the goldfish would provide an actively responsive enrichment option, the chimpanzees spent relatively little time interacting with the fish. Most of the fish-directed behavior was passive looking and did not significantly affect the chimpanzees' behavior in any way. The chimpanzees responded to the fish more in the first week, probably due to the initial novelty of the fish, than they did in the following three weeks. Increased use or interest in a variety of enrichment objects may be due to a "novelty effect,"26,27 which may have occurred in our study. The chimpanzees did seem to enjoy watching the fish being fed, and the younger female attempted to "feed" her fish by placing a biscuit on top of the aquarium on various occasions. Although the subjects of this study were individually housed, they live in complex environments with novel enrichment items and social interaction provided on a daily basis which may have contributed to their lack of interest in the goldfish.
In a study by Mason & Green,28 which investigated the response of rhesus macaques to another species, 12 monkeys were given access to an albino rat. The mean duration of contact ranged between 1.3 and 10.2 seconds/trial in the first phase of the study when contact was limited. In the second phase of the study when the monkeys actually lived with the rat, interaction and time spent in contact with the rat increased. The fact that the chimpanzees in this study could not actually contact the fish may have contributed to the lack of time spent interacting with them. In other studies where nonhuman primates were given direct access to a dog or fish, they spent more time interacting with them.19,20,24 Of course, direct access to the fish usually entailed their use as a food item and feeding enrichment has been shown to be particularly effective with nonhuman primates.11 We also encountered practical implementation problems with this enrichment option. When the chimpanzees displayed they oftentimes banged and shook the cage—resulting in a temporary loss of water in the aquariums or worse, cracks in the aquariums. Well meaning caretakers would sometimes try to refill these aquariums with chlorinated tap water which resulted in the death of the fish. The Plexiglas holders were also occasionally broken by the chimpanzees.
At the conclusion of this study the authors decided that it might be more beneficial to provide the goldfish on a more intermittent schedule in an attempt to alleviate the "novelty effect." This is currently being accomplished by having our enrichment technician schedule "fish" as an enrichment option one day each month. Transporting the fish on a daily basis rather than leaving them attached to the cage alleviates the problems of aquarium holders and loss of water. The authors also felt that the provision of a social group of fish might also be more interesting to the chimpanzees as well as being more enriching to the fish.
References
1. Brent, L., Lee, D.R. and Eichberg, J.W. (1989). The effects of single caging on chimpanzee behavior. Laboratory Animal Science 39: 345-346.
2. Fajzi, K., Reinhardt, V. and Smith, M.D. ( 1989). A review of environmental enrichment strategies for singly caged nonhuman primates. Lab Animal 10(2): 23-35.
3. Code of Federal Regulations. (1994). Animal Welfare 9 CFR 1.1:7-12.
4. Kessel, A. and Brent, L. Behavioural effects of transferring singly housed baboons to outdoor social groups. Proceedings of the Second International Conference on Environmental Enrichment, in press.
5. Reinhardt, V. ( 1994 ). Pair-housing rather then single-housing for laboratory rhesus macaques. Journal of Medical Primatology 23: 426-431.
6. Blackmore, W .M. ( 1989). Solution to psychological enhancement of the environment for the nonhuman primate. In E. F. Segal (Ed.), Housing, Care, and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications, Park Ridge, N.l., p. 235-243.
7. Kessel, A. and Brent, L. (1995). An activity cage for baboons, part I. Contemporary Topics in Laboratory Animal Science 34(6): 82-87.
8. Leu, M., Crockett, C.M., Bowers, C.L. and Bowden, D.M. (1993). Changes in activity levels of singly housed longtailed macaques when given the opportunity to exercise in a larger cage. American Journal of Primatology 30: 327(95)(Abstract).
9. Wolff, A. and Ruppert, G. (1991). A practical assessment of a nonhuman primate exercise program. Lab Animal 20(2): 36-39.
10. Bloomsmith, M.A., Keeling, M.E. and Lambeth, S.P. (1990). Videotapes: environmental enrichment for singly housed chimpanzees. Lab Animal 19(1): 42- 46.
11. Bloomsmith, M. A. (1989). Feeding enrichment for captive great apes. In: E. F. Segal (Ed.), Housing, Care, and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications, Park Ridge, N .1., p. 336-356.
12. Markowitz, H., Stevens, V .J., Mellen, J.D. and Barrow, B.C. (1981). Performance of a mandrill (Mandrillus sphinx) in competition with zoo visitors and computer on a reaction-time game. Acta Zoologia et Pathologica Antverpiensia 76: 169-180.
13. Snowdon, C.T. and Savage, A. (1989). Psychological well-being of captive primates: general considerations and examples from Callitrichids. In: E.F. Segal (Ed.), Housing, Care, and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications, Park Ridge, N.J., p. 75-88.
14. Washburn, D.A. and Rumbaugh, D.M. (1992). Investigations of rhesus monkey video-task performance: evidence for enrichment. Contemporary Topics in Laboratory Animal Science 31(5): 6-10.
15. Schapiro, S.J., Brent, L., Bloomsmith, M.A. and Satterfield, W.C. (1991). . Enrichment devices for nonhuman primates. Lab Animal 20(6): 22-28.
16. Struthers, E.J., Rodriguez, P., Cooper, P. and Rowell, J. (1990). Xenospecific enrichment at the Primate Research Institute. Laboratory Primate Newsletter 29(2): 14-15.
17. Coehlo, A.M. Jr. (1980). Guardian behavior by baboons towards felines. Laboratory Primate Newsletter 19(3): 1-10.
18. Capitanio, J.P. (1985). Early experience and social processes in rhesus macaques (Macaca mulatta): II. Complex social interaction. Journal of Comparative Psychology 99: 133-144.
19. Mason, W.A. and Kenney, M.D. (1974). Redirection of filial attachments in rhesus monkeys: dogs as mother surrogates. Science 183: 1209-1211.
20. Thompson, M.A., Bloomsmith, M.A. and Taylor, L.L. (1991). A canine companion for a nursery-reared infant chimpanzee. Laboratory Primate Newsletter 40(2): 1-4.
21. Vessels, J. (1985). Koko.s kitten. National Geographic 167(1), 110-113.
22. Bayne, K.A.L., Dexter, S.L. and Strange, G.M. (1993). The effects of food treat provisioning and human interaction on the behavioral welhbeing of rhesus monkeys(Macaca mulatta). Contemporary Topics in Laboratory Animal Science 32(2): 6-9.
23. Bloomsmith, M.A., Lambeth, S.P., Laule, G. and Thurston, R.H. (1993). Training as environmental enrichment for chimpanzees. American Journal of Primatology 30, 299 (Abstract).
24. King, J.E. and Norwood, V.R. (1989). Free-environment rooms as alternative housing for squirrel monkeys. In: E.F. Segal (Ed.), Housing, Care, and Psychological Wellbeing of Captive and Laboratory Primates. Noyes Publications, Park Ridge, N.l., p. 102-114.
25. Hamilton, W.J., III and Tilson, R.L. (1985). Fishing baboons at desert waterholes. American Journal of Primatology 8: 255-257.
26. Box, H.O. (1988). Behavioural responses to environmental change. Observations on captive marmosets and tamarins (Callitrichidae). Animal Technology 39(1): 9-16.
27. Nash, L.T. and Chilton, S.M. (1986). Space or novelty?: Effects of altered cage size on Galago behavior. American Journal of Primatology 10: 37-49.
28. Mason, W .A. and Green, P .C. ( 1962). The effects of social restriction on the behavior of rhesus monkeys: IV. Responses to a novel environment and to an alien species. Journal of Comparative and Physiological Psychology 55: 363-368.
Reproduced with permission of the Institute of Animal Technology.