V Reinhardt, C Liss and C Stevens
Animal Welfare Institute, P0 Box 3650, Washington, DC 20007, USA
Abstract
A review of the scientific literature gives evidence that transferring previously single-caged adult macaques to permanent compatible pair-housing arrangements (isosexual pairs, adult/infant pairs) is associated with less risk of injury and morbidity than transferring them to permanent group-housing arrangements. Juvenile animals can readily be transferred to permanent group-housing situations without undue risks. Safe pair formation and subsequent pair-housing techniques have been developed for female and male rhesus (Macaca mulatta), stump-tailed (M. arctoides) and pig-tailed macaques (M. nemestrina) as well as for female long-tailed Macaques (M. fascicularis). Pair housing does not jeopardize the animals' physical health but it increases their behavioural health by providing them with an adequate environment to satisfy their need for social contact and social interaction.
Keywords: aggression, animal welfare, behavioural health, compatibility, distress, morbidity, primates, psychological well-being, social housing, undernourishment
Published in Animal Welfare 4(4), 307-328 (1995)
© 1995 Universities Federation for Animal Welfare
This article has been reproduced with the permission of the publisher.
Introduction
The housing of non-human laboratory primates is a controversial issue. While the public argues that individual housing is not species-adequate and hence ethically not justifiable, some members of the primatological research community are reluctant to give up a housing system that is seemingly serving their scientific enterprise so well. A recent survey of North American primatological institutions showed that the most common laboratory primates, i.e. rhesus macaques (Macaca mulatta), long-tailed macaques (M. fascicularis), pig-tailed macaques (M. nemestrina), and stump-tailed macaques (M. arctoides), are being housed in appropriate social environments on average in only 38 per cent of cases (Reinhardt 1994a) despite the fact that published scientific information strongly supports guides and rules prescribing housing conditions that address the animals' social disposition.
The United States' Guide for the Care and Use of Laboratory Animals (National Institutes of Health 1985) states that group housing should be considered for communal animals. The British Code of Practice for the Housing and Care of Animals Used in Scientific Procedures (Home Office 1989) recommends that non-human primates be so housed that they have an opportunity for social interactions. The US Animal Welfare Act (US Department of Agriculture 1991) requires that institutions address the 'social needs' of social non-human primates. An animal may be exempt from social housing for health reasons and approved scientific research reasons. The Swiss Animal Protection Law (Der Schweizerische Bundesrat 1981) explicitly restricts such exemptions for health reasons. The Canadian Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care 1993) stipulates that non-human primates should be provided with a social environment conducive to their well-being. The International Primatological Society's Guidelines (International Primatological Society 1993) propose that unless absolutely essential, primates should not be housed alone in a cage on a long-term basis, because a compatible conspecific probably provides more appropriate stimulation to a captive primate than any other potential environmental enrichment factor.
The intent of the guides and rules reflects frequently published notions of primatologists. Chance et al (1983) argue that except for some specialized cases the accepted practice of housing monkeys singly is completely unjustified, since primatologists are fully aware that monkeys are social animals and require companions for a healthy life. With sociality so central to the very survival of primates (Bernstein 1991), Bramblett (1989) points out that the most stimulating, diverse and biologically important addition to the welfare of a captive primate is a social companion (cf. Bennett & Davis 1989; Fouts et al 1989; Pereira et al 1989; Segal 1989; Ruempler 1992). Social deprivation should not be considered any more normal than water or food deprivation (de Waal 1991). The assumption that non-human primates have social needs (US Department of Agriculture 1991) is echoed by Novak and Suomi (1991) who stated that social interaction is crucial for normal development in most primate species, and that having access to one or more companions may be the most effective way to foster their psychological well-being (cf. Novak & Drewsen 1989).
Some primatologists warn against social housing, underscoring an increased potential for the transmission of contagious diseases, for wounding and for undernourishment (Novak & Suomi 1988; cf. Vandenbergh 1989; Woolverton et al 1989). The concern for wounding has been expressed most strongly by Line (1987) arguing that any plan to increase social interaction also increases the risk of injury and death. Unless they have grown up in the same social group, primates are not likely to tolerate each other when placed together as adults (cf. Line et al 1989a). The fear of risking injury is shared by Coe (1991) cautioning that especially when new pairs are formed 'veterinarians will be kept quite busy suturing wounds'. Novak and Suomi (1988) underscored that stress may be increased in pairs as a result of incompatibility or excessive aggression by the dominant member of the pair. Ruppenthal et al (1991) pointed out that pair rearing may lead to behavioural maladaptation.
Taking both, the social disposition of primates and the potential risk of social housing into account, the Association of Primate Veterinarians strongly recommends that a program of social interaction be adopted by each institution (Keeling 1990). Contrary to this veterinary recommendation, only 12 per cent of primatologists (n = 105 respondents) indicated that if non-human primates should have rights, the provision of an appropriate social situation should be one of them (Petto 1994). Investigators at the National Institutes of Health suggest social housing of caged research primates less frequently (7.7%) as a modification of the cage environment, than provision of inanimate enrichment objects (27.8%) such as toys, swings, perches and shelves (National Institutes of Health 1991). Woolverton et al (1989) echoes this attitude by expecting only 'marginal benefits' of social housing for laboratory non-human primates.
The conflicting opinions of professionals working with laboratory primates warrant an evaluation of possible arguments used to justify individual housing (cf. Visalberghi & Anderson 1993). The present review focuses on studies conducted in macaques (Macaca spp.), because comprehensive published information is available only for this genus.
Aggression
Group housing
Group housing is probably the biologically most appropriate housing condition for macaques (cf. Bernstein 1991; Rolland 1991). Aggressive intolerance of strange conspecifics, however, distinguishes adult macaques; and the artificial formation of groups or the introduction of strangers into established groups is associated with considerable risks of trauma and death (rhesus macaques: Bernstein & Mason 1963; Southwick 1967; Bernstein et al 1974; Fairbanks et al 1977, pig-tailed macaques: Bernstein 1969; Tokuda & Jensen 1969; Erwin 1979, long-tailed macaques: Dollinger 1971, stump-tailed macaques: Rhine & Cox 1989). Kessler et al (1985), for example, routinely formed single male harems of 15-25 rhesus macaques and reported a 13 per cent trauma mortality rate per year.
Line et al (1990a) tried to circumvent the consequences of xenophobia in rhesus macaques. Future group members were therefore familiarized by placing two monkeys in wire-mesh cages at 7cm distance for 15 minutes. Fights were common during the first day of group formation. By day four, one male was depressed and withdrawn, and was regularly harassed by two of the other males. The victim was permanently removed for treatment of several bite wounds. Four days later, the top-ranking female was found dead in the cage from trauma. Within less than a month 77 per cent of the animals (10/13) had sustained injuries.
Assuming that adequate familiarization of potential group members cannot be achieved in the course of brief non-contact encounters, Reinhardt (1991a) formed two isosexual groups of six adult rhesus macaques each, after group members had been given the opportunity to physically interact with each other on a one-to-one basis for one week. In both instances, group incompatibility was heralded by certain subjects challenging other individuals to whom they had originally been subordinate. Aggressive harassment was intensive and persistent, but victims showed no resistance. Both groups were split up within less than one hour to avoid similar consequences as those described by Line et al (1990a).
Unlike adults, socially experienced juvenile and subadult macaques can readily be transferred from single-housing to group-housing arrangements with other peers. Bernstein and Draper (1964) released eight female and three male juvenile rhesus macaques into a compound and observed that the animals formed an organized social group without resorting to serious aggression. Schapiroet al (1994) reported no problems associated with aggression when moving 19 juvenile rhesus macaques from single cages into heterosexual peer groups and maintaining group membership until the animals reached sexual maturity. Wolff and Ruppert (1991) encountered no aggression-related problem when forming a heterosexual group of six formerly single-caged subadult rhesus macaques. Group members were compatible and showed no serious aggression during a nine-week follow-up period.
Vicious fighting among adult macaques is a frustrating management problem not only when groups are artificially formed, but also when excessive aggression develops spontaneously in well-established troops (Chance et al 1977; Ehardt & Bernstein 1986; Samuels & Henrickson 1983; Reinhardt et al 1987a; Rolland 1991) and is likely to flare up in association with sexual competition (Judge et al 1994; Schapiro et al 1994) and whenever changes are being made in the troop's composition (Erwin 1977; Fairbanks et al 1978; Kaplan et al 1980; Kessler et al 1985; Rolland 1991).
Pair housing
Pair-housing techniques have been developed to avoid the risk of injury attendant on group housing. Reinhardt et al (1988a) and Eaton et al (1994) tested pairs of previously single-caged adult female rhesus macaques. Partners were first familiarized with each other in double cages with transparent barriers. Subsequent pair formation was successful in 89 per cent (16/18) and 90 per cent (19/21) of cases. In both studies two pairs were separated because of fighting. Eaton et al (1994) as well as Reinhardt et al (1988a) ascertained partner compatibility (cf. Table 2) in 83 per cent of cases for 36 and 4 months respectively (Table 1).
In an attempt to minimize the typical fighting during the initial introduction of partners (cf. Maxim 1976), Reinhardt (1989) paired adult male rhesus macaques after it was verified that partners had established clear dominance-subordination relationships during a five-day period of non-contact familiarization. It was hypothesized that the establishment of such rank relationships would make aggressive disputes rather unnecessary during pair formation (cf. Bernstein & Gordon 1974), because partners would respect their relative dominance-subordination relationships. In order to form five pairs with clear relationships, seven different dyads had to be screened. No rank decisive interactions (cf. Table 2) could be observed in two dyads. The partners of the other five dyads established rank relationships within the first day of familiarization. When those males were paired in a different double cage (a precaution against possible territorial antagonism: Reinhardt et al 1988a; Line et al 1990a) they confirmed their rank relationships within the first six minutes. No fighting, no biting and no signs of aggressive harassment or depression were observed during a five-day follow-up period. The males were strictly housed in male-only areas to exclude the risk of sexual competition possibly triggered by the sight of females (cf. Coe & Rosenblum 1984; Coe 1991). Reinhardt (1994c, d) tested this technique again in other rhesus as well as in stump-tailed macaques of both sexes with the following results:
- Male rhesus pairs were compatible in 80 per cent of cases throughout follow-up periods of one to five years (Table 1).
- Female rhesus pairs were compatible in 88 per cent of cases during follow-up periods of one to seven years (Table 1).
- All stump-tailed pairs were compatible during pair formation and during a six-month follow-up period (Table 1).
Crockett et al (1994) formed isosexual pairs of adult long-tailed macaques. Potential partners were also pre-familiarized, but no attempt was made to ascertain that they had established clear dominance-subordination relationships before introduction. During pair formation 13 per cent of the female pairs, and 67 per cent of the male pairs engaged in fighting. Two male dyads were split due to serious injuries. Unlike the above mentioned studies, all newly paired subjects of the study by Crockett et al (1994) were separated after 90 minutes and reunited on the following day. On days 2-13 each pair was separated daily for 17 hours and re-introduced thereafter. All female pairs but only 40 per cent of the male pairs were compatible throughout the two-week study period (Table 1). Clarke et al (1986) also formed pairs of male long-tailed macaques, but allowed partners to continuously stay together as long as they did not fight excessively. Pairs were compatible in 58 per cent of cases for an eight-month follow-up period. Unfortunately, the authors did not elaborate on how partners were initially introduced to each other.
Table 1: Success rates of pairing techniques for previously single-caged adult macaques.
| Pairing technique | Compatibility | Follow-up | Subject Macaca | Reference |
|---|---|---|---|---|
| prefamiliarization | 83% (15/18) | 4-6 months | female mulatta | Reinhardt et al 1988a |
| 92% (11/12) | 36 months | female mulatta | Eaton et al 1994 | |
| 75% (3/4) | 5-6 months | female fascicularis | Line et al 1990a | |
| 100% (15/15) | 2 weeks | female fascicularis | Crockett et al 1994 | |
| 40% (6/15) | 2 weeks | male fascicularis | Crockett et al 1994 | |
| unknown | 58% (7/12) | 8 months | male fascicularis | Clarke et al 1986 |
| rank relationships established during prefamiliarization | 100% (5/5) | 5 days | male mulatta | Reinhardt 1989 |
| 80% (16/20) | 1-5 years | male mulatta | Reinhardt 1994d | |
| 88% (68/77) | 1-7years | female mulatta | Reinhardt 1994d | |
| 100% (5/5) | 6 months | female arctoides | Reinhardt 1994c | |
| 100% (3/3) | 6 months | male arctoides | Reinhardt 1994c | |
| no familiarization | 94% (16/17) | 7-11 months | female/infant mulatta | Reinhardt et al 1987b |
| 94% (61/65) | 1-8 years | female/infant mulatta | Reinhardt 1994d | |
| 92% (11/12) | 7-11 months | male/infant mulatta | Reinhardt et al 1987b | |
| 92% (12/13) | 1-4 year | male/infant mulatta | Reinhardt 1994d |
Successful isosexual pair formation has been reported for male and for female pig-tailed macaques but the actual technique of partner introduction has not been published (Reinhardt 1994a).
As an alternative to adult-adult pairings, Reinhardt et al (1987b) socialized previously single-caged rhesus macaques with 1-1.5 year-old, naturally weaned infants from breeding troops. It was hypothesized that infants of this age would trigger parental responses rather than overt aggression in the adults (cf. Lorenz 1971; Redican & Mitchell 1973; Gibber & Goy 1985; Schwind et all992). Infants were therefore directly placed into the cages of adults of both sexes. Pairs were compatible in 90 per cent of cases, with the adult subject huddling with the introduced infant, and the latter showing no signs of injury or depression. Compatibility was 94 per cent for female-infant pairs, 83 per cent for male-infant pairs (Table 1).
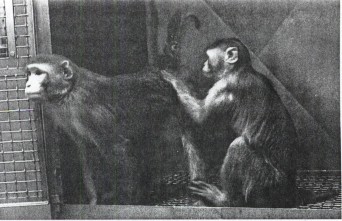
Adult males were as affectionate with juvenile companions as adult females (Figure 1). Pair incompatibility was due to non-injurious aggression in two cases, a non-bleeding injury in one case. In an earlier study, Reinhardt (1994c) ascertained one to eight-year compatibility in 94 per cent of female-infant pairs, one to four-year compatibility in 92 per cent of male-infant pairs (Table 1)

There is little information about the risk of forming pairs of unfamiliar young macaques. Brandt and Mitchell (1973) paired eight pre-adolescent rhesus macaques with eight infants in isosexual and heterosexual dyads without encountering aggression related problems during a three-week follow-up period. Schapiro et al (1993) transferred 64 unfamiliarized juvenile rhesus macaques from single-housing to heterosexual pair-housing conditions for one year without noteworthy problems associated with aggression (cf. Schapiro et al 1991; Schapiro & Bushong 1994). Weaned rhesus infants have been housed in isosexual pairs routinely at the Wisconsin Regional Primate Research Center without any aggression related problems. When this manuscript was written, the Center had 12 female and 4 male pairs. Partners had been directly introduced to each other with no incidence of serious aggression (cf. Reinhardt 1994d), and they had lived together as compatible companions for up to three years (Figure 2).
Morbidity
Schapiro and Bushong (1994) assessed the rates of veterinary treatment in 98 rhesus macaques under the conditions of single, pair and group housing. Daily treatment per monkey was highest in the group condition, lowest in the pair condition, intermediate in the single condition. The authors underlined that there was relatively little intervention needed for pair-housed animals due to less diarrhea and little trauma. Reinhardt (1990a) compared rates of veterinary treatment per year of 237 individually-housed with that of 382 pair-housed rhesus macaques that were kept in the same facility. Treatment was required by 23 per cent (54/237) of the single-housed, but only by 10 per cent (38/382) of the pair-housed subjects. Eaton et al (1994) found no significant difference in rates of clinical morbidity in 12 single-housed and 24 pair-housed female rhesus macaques.
Distress
There is no scientific evidence demonstrating that living in a group per se causes more distress than living alone. Living with conspecifics, however, may provide a buffer against environmental stress that the singly caged subject is lacking (cf. Bovard 1959; Rowell & Hinde 1963; Epley 1974; Cubicciotti & Mason 1975; Cobb 1976; Arnone & Dantzer 1980; Gunnar et al 1980; Taylor 1981; Coc et al 1982; Gonzalez et al 1982; Hennessy 1984; Stanton et al 1985; Mendoza & Mason 1986; Lyons et al 1988; de Monte et al 1992). Gust et al (1994), for example, removed seven adult female rhesus macaques from their home group and housed them in a novel environment both alone or with a member of the group. Subjects experienced measurable distress (elevated cortisol concentrations and decrease in absolute number of lymphocyte subsets) in both conditions, but recovered from it significantly quicker in the presence of the companion. Shively et al(1989) noted in adult female long-tailed macaques that single housing may be a greater risk factor for atherogenesis than group housing. Thirty individually caged subjects had significantly moreextensive atherosclerosis in the coronary arteries than 47 group-housed subjects. Atherosclerosis extent was four times greater in animals that were kept alone than in those that were living in groups. Coelho et al (1991) assessed the effect of companionship in four baboons (Papio spp.) during a distressing restraint situation. The animals were tested under traditional single housing, and under experimental social housing which implied that subjects had visual, tactile and auditory contact with compatible conspecifics. Being restrained in company with familiar social partners resulted in significantly lower resting blood pressure and lower heart rates than when being restrained alone, suggesting that familiar companionship ameliorated physiological stress responses.
Eaton et al (1994), Crockett et al (1994) and Reinhardt (1994c) emphasized that grooming is the salient social behaviour of compatible macaque pairs, and that companions show agonistic interactions only rarely but have a strong preference to stay in close proximity to one another (cf. Washburn et al 1994). These observations indicate that compatible companionship is a source of comfort rather than distress.
Schapiro et al (1993) were unable to detect significant differences in cortisol response to single versus pair housing in 64 juvenile rhesus macaques. This finding supports results of Reinhardt et al (1991) who examined serum cortisol concentrations of single-housed and compatible pair-housed adult rhesus macaques. In both sexes, cortisol concentrations of isosexually paired animals (ten females, ten males) showed no significant differences with those of single animals (five females, five males). Both in female and in male pairs, dominant partners had cortisol concentrations that were equivalent to those of their subordinate counterparts (females: 19.5 µg dl-1 vs 19.4 µg dl-1, males: 17.5 µg dl-1 vs 17.2 µg dl-1). These data corroborate those of Crockett et al (1994) who found no evidence of elevated levels of urinary cortisol in response to compatible pair housing versus single housing in ten adult female long-tailed macaques.
Eaton et al (1994) assessed immune stress responses in adult female rhesus macaques when being single-housed (n = 45) versus pair-housed (n = 24) with a compatible partner. Lymphocyte proliferation response did not decline after pairing and showed no difference between dominant and subordinate members of pairs. The analysis of behavioural profiles complemented this finding, suggesting that subordinates were not stressed by the experience of pairing. Coe (1991) reported a decrease in lymphocyte proliferation response in old rhesus macaques when being transferred from single housing to pair or group housing with juveniles. These physiological observations are in line with ethological records by Reinhardt and Hurwitz (1993) showing that pair-housed aged rhesus macaques have to discipline their sometimes all too frisky young companions. The authors therefore recommended that pairs should be split in the event of excessive disturbance of the aged subjects by their young cagemates.
Taking the expression of gross behavioural disorders as signs of distress, compatible companionship may have a therapeutic effect (Harlow & Suomi 1971; Brandt & Mitchell 1973; Bushong et al 1992). Line et al (1990b) observed self-abusive behaviours in five female long-tailed macaques and noted cessation of this disorder in all cases after the animals had been transferred to compatible pair housing for five to six months. Reinhardt et al (1987b) noted bizarre stereotypical behaviour patterns in three singly-housed adult female rhesus macaques. All three animals were paired with infants and gradually abandoned their peculiar habits within four months of social housing. Bloomsmith and Schapiro (1994 personal communication) noted that pair housing previously single-caged juvenile rhesus macaques, significantly reduced the percentage of time that was spent by the subjects engaged in self-aggressive activities. Ruppenthal et al (1991) observed stereotypies in individually caged but not in pair-housed pig-tailed infants.
Social housing need not be distressing for laboratory non-human primates, however, involuntary separation from familiar companions for routine management or experimental reasons may be a disturbing experience (Redican & Mitchell 1973; Willott & McDaniel 1974; Suomi et al 1975; Reite et al 1981; Rasmussen & Reite 1982; Coe 1991; Mendoza 1991; Gordon et al 1992), as is the involuntary removal from the familiar home-cage (Mitchell & Gomber 1976; HoIm 1979; Line et al 1989b; Line et al 1991). Allowing partners continually to keep visual and/or auditory contact during physical separation is likely to minimize their stress response (cf. Gust et al 1994; Table 2).
Table 2: Ethological guidelines to avoid aggression during and after pair formation of previously single-caged adult macaques of the same sex.
| 1. | Allow potential partners to establish clear dominance-subordination relationships during a non-contact familiarization period. This is a basic condition so that the animals will be able to live together in harmony. |
| 2. | Check for signs of an established rank relationship, such as unidirectional fear-grinning, withdrawing, looking away, threatening-away and absence of reciprocal threatening. |
| 3. | Pair partners only after they have established their rank relationship. This will give them no reason to fight over dominance. |
| 4. | Pair partners in a different double cage. This avoids possible territorial antagonism. |
| 5. | Do not force animals to live together when they are incompatible. Signs of incompatibility are serious injury, persistent fighting, aggressive harassment, depression and inadequate food sharing. |
| 6. | Keep male pairs in male-only areas. This avoids sexual competition possibly triggered by the sight of females. |
| 7. | Let a new pair live together continuously for at least one month. This allows them to establish a stable social relationship. |
| 8. | If partners have to be physically separated thereafter, allow them to keep continual visual and auditory contact. This minimizes the possible stress associated with separation. |
| 9. | If partners have to be housed in different rooms for more than one week, do not simply re-unite them in their home-cage thereafter, but give them the opportunity to briefly recognize each other across a temporary transparent cage divider. This is a safeguard that the animals will not treat each other as strangers, ready to fight over dominance. |
| 10. | Never threaten or scare the animals. This could excite them so much that they redirect the triggered aggressive tension toward each other. |
Undernourishment
Eaton et al (1994) compared body weight developments of adult female rhesus macaques and found no differences between single-housed (n = 12) versus pair-housed subjects (n = 24), nor between dominant and subordinate partners of compatible pairs. Reinhardt et al (1988b) assessed body weight developments of 28 adult female rhesus macaques in the month before pairing, and in the first two months after formation of 14 compatible pairs. Compared with the pre-pairing situation, dominant partners showed no significant change in body weight during the first two months, while subordinates exhibited a significant increase in weight in the second month. Reinhardt and Hurwitz (1993) recorded body weights of eight aged rhesus macaques (six females, two males) one year prior to being paired with compatible companions, at the day of pairing, and again one year after pairing. The aged animals were so old (31-36 years) that they experienced a gradual loss in body weight. Living with a companion did not accelerate this biological process: average yearly body weight balances were -4.4 per cent in the year prior to pairing, -4.2 per cent in the year after pairing. Reinhardt et al (1987b, 1989) include food sharing between partners as one criterion of pair compatibility to guarantee that subjects obtain their adequate shares of the daily food ration (cf. Table 2).
Maladaptation
Chamove et al (1973) examined eight rhesus infants raised without a mother and demonstrated that allowing them to interact with only each other precludes normal social behaviour development. Familiar peers exhibited a preponderance of mutual clinging because they had no opportunity to develop affectional ties with any other conspecifics (Chamove 1973). Ruppenthalet al (1991) tested infant pig-tailed macaques during play sessions scheduled throughout the first eight postnatal months. Play groups consisted each of two females and two males: four pair-housed versus four individually housed subjects. The animals had been separated from their mothers shortly after birth. They were artificially reared during 14-28 days and subsequently assigned to the experimental protocol. During the play sessions, pair-housed infants tried to maintain physical contact with their partners by clinging to each other. Individually housed infants spent significantly less time clinging to playmates and were less afraid to examine them. No significant difference in social play was found between rearing conditions. Unlike single subjects, paired subjects exhibited no rock/huddle/self clasp and stereotypic behaviour patterns, but instead spent significantly more time playing with toys. At the end of the study, all animals were placed in cages with seven to eight other monkeys. Informal observations suggested that the pair-reared subjects fared poorly in this social housing situation: they were submissive and appeared depressed. These reactions were not seen in the individually reared subjects. The authors concluded that pair rearing yields abnormal social development in pig-tailed macaques.
Unlike motherless peer rearing, mother rearing and subsequent pair housing with another peer is unlikely to produce developmental disorders. Schapiro et al (1994) separated 24 mother-reared rhesus infants from their natal group when they were a little over one-year-old and placed them in single cages for one year. The animals were subsequently paired with another similarly reared peer of the opposite sex. When they were three-year old, subjects were placed into groups of six to eight other monkeys. The authors did not observe any social integration problems on these occasions.
Discussion
The present review leads to the conclusion that arguments justifying individual caging of laboratory non-human primates may often be based on assumptions rather than on facts. Rhesus macaques for example, are commonly single-housed because it is generally believed that the species is particularly. aggressive and hence unsuitable for social housing. Disregarding this conventional wisdom, 295 adult rhesus macaques of both sexes assigned to research, were successfully transferred from single housing to permanent isosexual pair housing with each other (102 pairs) or to pair housing with infants (91 pairs). This socialization program was associated with serious, yet not life threatening wounding in only less than 1 per cent (3/386) of animals (Reinhardt 1991b).
The available information indicates that transferring single-caged macaques to group housing is likely to be associated with a relatively high risk. The inherent socio-ethological advantages of group living, however, warrants carefully controlled and monitored attempts to provide compatible group housing, especially for young animals.
Scientific findings show that pair formation and subsequent permanent pair housing offers a safe alternative to unsuccessful group housing attempts. The relatively high degree of aggressive incompatibility found in male long-tailed macaques (Crockett et al 1994; cf. Goosen et al 1984; Whitney & Wickings 1987) could probably be attenuated as in rhesus and stump-tailed males (Reinhardt 1994c,d), if partners were allowed: a) to establish rank relationships during prefamiliarization, and b) to stay together continuously rather than intermittently thereafter. Future studies will also have to examine if adult-infant pairing is equally successful in other macaque species as it is in rhesus macaques.
The information regarding the impact of social housing on morbidity is limited, but strongly suggests that the health risks associated with group living can effectively be minimized or even neutralized when non-human primates are housed in compatible pairs.
The literature reviewed offers no evidence that compatible social housing causes more distress than single housing. This does not imply that single housing is stressful. Compatible companionship, however, unlike solitary confinement, functions as a buffer against stress during fear-inducing events associated with routine management practices and experimental procedures. Housing gregarious non-human primates in compatible social conditions is also a safeguard against the pathological condition of behavioural disorders so commonly seen in single-caged subjects (cf. Erwin et al 1973). Goosen et al (1984) recommend therefore that individual housing should be used only when strictly necessary for the well-being of the animals, e.g. during recovery from surgery.
To make social housing a successful management improvement:
- partner compatibility must be ascertained on a daily basis (cf. US Department of Agriculture 1991).
- animals should not be forced to live with each other if observable and/or measurable evidence indicates that they are incompatible (cf. US Department of Agriculture 1991).
- companions should have the option of moving into temporary visual seclusion (Goosen et al 1984; Whitney & Wickings 1987; O'Neill 1989; Taff & Dolhinow 1989; Reinhardt & Reinhardt 1991).
- male companions should be housed in male-only areas to prevent sexual competition (Reinhardt 1992a).
- social relationships should not be disrupted to avoid possible distress triggered by involuntary separation from familiar conspecifics (cf Gordon et all 992).
It has been shown that groups/pairs can be successfully trained to voluntarily separate for common procedures such as blood collection (Bunyak et al 1982; Vertein & Reinhardt 1989; Clarke et al 1990; Reinhardt & Cowley 1992), feces collection (Phillippi-Falkenstein & Clarke 1992), systemic drug administration (Reinhardt 1992c), topical drug application (Reinhardt & Cowley 1990), vaginal swabbing (Bunyak et al 1982), tethering (Reinhardt 1991b), and headcap implantation (Reinhardt 1991b). Socially housed animals can readily be conditioned to allow capture in transport boxes (Smith 1981; Boccia et al 1992; Reinhardt 1992b; Luttrell et al 1994). If an animal has to be kept singly for a limited time period (i.e. metabolic studies, feeding studies, urine collection, post-operative recovery, experiments involving chair restraint) a compatible companion can be kept close by behind a transparent barrier allowing visual and/or acoustic social contact (cf. Reinhardt et al 1989; Coelho et al 1991).
The normal social adjustability by pair-housed macaque infants that were naturally raised by their mothers, as opposed to the relatively poor social adjustment of pair-housed infants that were artificially reared without mother contact, endorses natural rather than artificial rearing conditions for non-human primates. The unnatural attachment of a mother-deprived infant to another infant inhibits rather than facilitates the development of normal peer-peer interaction (Chamove et al 1973). The cause of this behavioural problem is obviously not the social peer-housing condition but the absence of the biological mother (cf. Mason 1991). This notion is supported by findings of Alexander (1966) who reared infant rhesus macaques from birth for eight months with only their mothers. These animals did not show the typical together-together syndrome when separated from their mothers, and socialized with peers.
The present survey of the literature supports the regulatory recommendations of housing gregarious non-human primates, such as macaques, in an environment that allows them to express their social disposition. Keeping macaques under social rather than single housing conditions provides a simple way of approximating conditions that are normal. It makes the animals more valuable for unbiased scientific research because they are now truly what they are supposed to be: social animals. Partners of compatible macaque pairs spend approximately 1/5 of their time interacting with each other in affiliative ways typical for the species (Ranheim & Reinhardt 1989; Reinhardt 1990b; Line et al 1990a; Reinhardt & Hurwitz 1993; Crockett et al 1994; Eaton et al 1994; Reinhardt 1994c; Schapiro & Bloomsmith 1994). This is compatible with the situation in groups containing animals of both sexes and different ages (Rhine & Kronwetter 1972; Post & Baulu 1978; Bernstein 1980; Teas et al 1980; O'Keeffe & Lifshitz 1985; Chopra et al 1992; Leon et al 1993), and suggests that being transferred from single to pair housing improves the animals' behavioural health by providing them with an appropriate environment for the expression of their social disposition (Reinhardt 1987). Pair housing is likely to be one of the least expensive and most effective alternatives for improving the welfare of macaques in research facilities (Line et al 1990b).
It would defeat the purpose of the regulations to stubbornly force laboratory macaques to live together and possibly kill each other. Given the complexity of non-human primates and the inherent dynamics of their social relationships, it would be unrealistic to expect unvarying compatibility (Reinhardt 1994b). No strict rule can therefore be set which will guarantee successful social housing in all instances. Attempts to transfer single-caged animals to compatible permanent group or pair housing have to be based on ethological principles (Table 2), common sense, some expertise, and also on good will in order to take variables into account that may directly affect the outcome. Such variables are: technique of partner introduction, sex, age, rearing history, social experience, health status, research protocol, animal caregiver/technician, feeding regime and physical environment.
The published data show that previously single-caged macaques can be transferred to social housing adequate for the species (group housing for juveniles, pair housing for adults) without undue risks to individual animals. Techniques that are currently applied successfully with macaques should be attempted with other appropriate species and modified if necessary. The work described in this review presents a justifiable plan of action to provide social nonhuman primates with a social rather than solitary housing environment.
Animal welfare implications
Scientific evidence shows that laboratory macaques can be permanently housed in a compatible social environment without unduly jeopardizing their safety. Providing them with a social rather than the traditional solitary environment, fosters their well-being by offering them means to satisfy their need for social interaction and social contact.
Acknowledgements
We are thankful to Mollie Bloomsmith, Gray Eaton, Catherine Reinhardt and Annie Reinhardt for reading the first draft of this manuscript and offering constructive criticism. The manuscript also benefited from very helpful suggestions of three anonymous referees.
References
Alexander B K 1966 The effects of early peer-deprivation on juvenile behaviour of rhesus monkeys. Doctoral Dissertation, University of Wisconsin, USA
Arnone M and Dantzer P 1980 Does frustration induce aggression in pigs? Applied Animal Ethologv 6: 351-362
Bennett C L and Davis R T 1989 Long term animal studies. In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 213-234. Noyes Publications: Park Ridge, USA
Bernstein I S 1969 Introductory techniques in the formation of pigtail monkey troops. Folia Primatologica 10: 1-19
Bernstein I S 1980 Activity patterns in a stumptail macaque group (Macaca arctoides). Folia Primatologica 33.. 20-45
Bernstein I S 1991 Social housing of monkeys and apes: group formations. Laboratory Animal Science 41: 329-333
Bernstein I S and Draper W A 1964 The behaviour of juvenile rhesus monkeys in groups. Animal Behaviour 12: 28-31
Bernstein I S and Gordon T P 1974 The function of aggression in primate societies. American Scientist 62: 304-311
Bernstein I S, Gordon T P and Rose R M 1974 Factors influencing the expression of aggression during introduction to rhesus monkey groups. In: Holloway R L (ed) Primate Aggression, Territoriality and Xenophobia pp 211-240. Academic Press: New York, USA
Bernstein I S and Mason W A 1963 Group formation by rhesus monkeys. Animal Behaviour 11: 28-31
Boccia M L, Broussard C, Scanlan J and Laudenslager M L 1992 Practice makes predictable: the differential effect of repeated sampling on behavioural and physiological responses in monkeys. In: Davis H and Balfour A D (eds) The Inevitable Bond: Examining Scientist-Animal Interactions pp 153-170. Cambridge University Press: Cambridge, UK
Bovard E W 1959 The effects of social stimuli on the response to stress. Psychological Review 66: 267-277
Bramblett C A 1989 Enrichment options for guenons in the laboratory. American Journal of Primatology Supplement 1: 59-63
Brandt E M and Mitchell G 1973 Pairing preadolescents with infants (Macaca mulatta). Developmental Psychology & 222-228
Bunyak S C, Harvey N C, Rhine R J and Wilson M 11982 Venipuncture and vaginal swabbing in an enclosure occupied by a mixed-sex group of stumptailed macaques (Macacca arctoides). American Journal of Primatology 2: 201-204
Bushong D, Schapiro S J and Bloomsmith M A 1992 Self-aggression in non-human primates: a review of its development/possible causes, methods of therapeutic treatment, and its relevance to the zoo situation. American Association of Zoological Parks and Aquariums (AAZPA) Regional Proceedings: 723-729
Canadian Council on Animal Care (CCAC) 1993 Guide to the Care and Use of Experimental Animals, Volume 1, 2nd edition. CCAC: Ottawa, Canada
Chamove A S 1973 Rearing infant rhesus together. Behaviour 47: 48-66
Chamove A S, Rosenblum L A and Harlow H F 1973 Monkeys (Macaca mulatta) raised only with peers. A pilot study. Animal Behaviour 21: 316-325
Chance R M A, Byrne B and Jones E 1983 A tandem cage for individually handling group-living monkeys. Laboratory Animals 17: 129-132
Chance R M A, Emory G R and Payne R G 1977 Status referents in long-tailed macaques (Macaca fascicularis): precursors and effects of a female rebellion. Primates 18: 611-632
Chopra P K, Seth S and Seth P K 1992 Behavioural profile of free-ranging rhesus monkeys. Primate Report 32: 75-105
Clarke M R, Kaplan J R, Bumsted P T and Koritnik D R 1986 Social dominance and serum testosterone concentration in dyads of male Macaca fascicularis. Journal of Medical Primatology 15:419-432
Clarke M R, Phillippi K M, Falkenstein J A, Moran E A and Suomi S J 1990 Training corral-living rhesus monkeys for fecal and blood sample collection. American Journal of Primatology 20:181 (Abstract)
Cobb S 1976 Social support as a moderator of life stress. Psychosomatic Medicine 38: 300-314
Coe C L 1991 Is social housing of primates always the optimal choice? In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 78-92. American Psychological Association: Washington, USA
Coe C L, Franklin D, Smith E R and Levine 5 1982 Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology and Behavior 29: 1051-1057
Coe C L and Rosenblum L A 1984 Male dominance in the bonnet macaque: a malleable relationship. In: Barchas P and Mendoza SP (eds) Social Cohesion: Essays Toward a Sociophysiological Perspective pp 31-64. Greenwood Press: Westport, USA
Coelho A M, Carey K D and Shade R E 1991 Assessing the effects of social environment on blood pressure and heart rates of baboon. American Journal of Primatology 23: 257-267
Crockett C M, Bowers C L, Bowden D M and Sackett G P 1994 Sex differences in compatibility of pair-housed adult long-tailed macaques. American Journal of Primatology 32: 73-94
Cubicciotti D D and Mason W A 1975 Comparative studies of social behavior in Callicebus and Saimiri: male-female emotional attachments. Behavioral Biology 16: 185-197
de Monte M, Anderson, J R and Charbonnier H 1992 Self-aggression in stumptail macaques: effects of frustration and social partners. Primates 33: 115-120
Der Schweizerische Bundesrat 1981 Tierschutzverordnung, Stand am 1. Januar 1992. Bundesrat: Bern, Switzerland
de Waal F B M 1991 The social nature of primates. In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 67-77. American Psychological Association: Washington, USA
Dollinger P 1971 Tod durch Verhalten bei Zootieren. Juris Druck und Verlag: Zurich, Switzerland
Eaton G G, Kelley S T, Axthelm M K, Iliff-Sizemore S A and Shugi S M 1994 Psychological well-being in paired adult female rhesus (Macaca mulatta). American Journal of Primatology 33:89-99
Ehardt C A and Bernstein I 5 1986 Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology 7: 157-181
Epley W 1974 Reduction of the behavioral effects of aversive stimulation by the presence of companions. Psychological Bulletin 81: 271-283
Erwin J 1977 Factors influencing aggressive behavior and risk of trauma in pigtail macaque (Macaca nemestrina). Laboratory Animal Science 27: 541-547
Erwin J 1979 Aggression in captive macaques: interaction of social and spatial factors. In: Erwin J, Maple T and Mitchell G (eds) Captivity and Behavior pp 139-171. Van Nostrand: New York, USA
Erwin J, Mitchell G and Maple T 1973 Abnormal behavior in non-isolate-reared rhesus monkeys. Psychological Reports 33: 515-523
Fairbanks L A, McGuire M T and Kerber W T 1977 Sex and aggression during rhesus monkey group formation. Aggressive Behavior 3: 241-249
Fairbanks L A, McGuire M T and Kerber W T 1978 Effects of group size, composition, introduction technique and cage apparatus on aggression during group formation in rhesus monkeys. Psychology Reports 42: 327-333
Fouts R S, Abshire M L, Bodamer M and Fouts D H 1989 Signs of enrichment: towards the psychological well-being of chimpanzees. In: Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 376-388. Noyes Publications: Park Ridge, USA
Gibber J R and Goy R W 1985 Infant-directed behavior in young rhesus monkeys: sex differences and effects of prenatal androgens. American Journal of Primatology 8: 225-237
Gonzalez C A, Coe C L and Levine S 1982 Cortisol responses under different housing conditions in female squirrel monkeys. Psychoneuroendocrinolgy 7: 209-216
Goosen C, van der Gulden W, Rozemond H, Balner H, Bertnes A, Boot R, Brinkert J, Dienske H, Janssen G, Lammers A and Timmermans P 1984 Recommendations for the housing of macaque monkeys. Laboratory Animal 1& 99-102
Gordon T P, Gust D A, Wilson M E, Ahmed-Ansari A, Brodie A R and McClure H M 1992 Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiology and Behavior 51: 467-472
Gunnar M R, Gonzalez C A and Levine S 1980 The role of peers on modifying behavioral distress and pituitary-adrenal response to a novel environment in year-old rhesus monkeys. Physiology and Behavior 25: 795-798
Gust D A, Gordon T P, Hambright M K and Wilson M E 1994 Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys. Hormones and Behavior 27:318-331
Harlow H F and Suomi S J 1971 Social recovery by isolation-reared monkeys. Proceedings of the National Academy of Science 68: 1534-1538
Hennessy M B 1984 Presence of companion moderates arousal of monkeys with restricted social experience. Physiology and Behavior 33: 693-69
Holm R A 1979 Effect of parental risk and prenatal stress on pregnancy outcome. In: Ruppenthal G C (ed) Nursery Care of Non-human Primates pp 21-26. Plenum Press: New York, USA
Home Office 1989 Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. HMSO: London, UK
International Primatological Society 1993 IPS International Guidelines for the Acquisition, Care and Breeding of Nonhuman Primates. Primate Report 35: 3-29
Judge P G, de Waal F B M, Paul K S and Gordon T P 1994 Removal of a trauma-inflicting alpha matriline from a group of rhesus macaques to control severe wounding. Laboratory Animal Science 44: 344-350
Kaplan J R, Manning P and Zucker E 1980 Reduction of mortality due to fighting in a colony of rhesus monkeys (Macaca mulatta). Laboratory Animal Science 30: 565-570
Keeling M A 1990 A historical view. In: Mench J A and Krulisch L (eds) Well-Being of Nonhuman Primates in Research pp 12-14. Scientists Center for Animal Welfare: Bethesda, USA
Kessler M J, London W T, Rawlins R G, Gonzales J, Martines H S and Sanches J 1985 Management of a harem breeding colony of rhesus monkeys to reduce trauma-related morbidity and mortality. Journal of Medical Primatology 14. 91-98
Leon S, Taylor L and Sussman R W 1993 Activity patterns of captive and free-ranging long-tailed macaques (Macaca fascicularis). Proceedings of the American Association of Zoological Parks and Aquariums Annual Conference: 171-178
Line S W 1987 Environmental enrichment for laboratory primates. Journal of the American Veterinary Medical Association 190: 854-859
Line S W, Markowitz H, Morgan K N and Strong S 1989a Evaluation of attempts to enrich the environment of single-caged non-human primates. In: Driscoll J W (ed) Animal Care and Use in Behavioral Research: Regulation, Issues, and Applications pp 103-117. Animal Welfare Information Center National Agricultural Library: Beltsville, USA
Line S W, Markowitz H, Morgan K N and Strong S 1991 Effect of cage size and environmental enrichment on behavioral and physiological responses of rhesus macaques to the stress of daily events. In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 160-179. American Psychological Association: Washington, USA
Line S W, Morgan K N, Markowitz H, Roberts J and Riddell M 1990b Behavioral responses of female long-tailed macaques (Macaca fascicularis) to pair formation. Laboratory Primate Newsletter 29(4): 1-5
Line S W, Morgan K N, Markowitz H and Strong S 1989b Heart rate and activity of rhesus monkeys in response to routine events. Laboratory Primate Newsletter 28(2): 9-12
Line S W, Morgan K N, Roberts J A and Markowitz H 1990a Preliminary comments on resocialization of aged rhesus macaques. Laboratory Primate Newsletter 29(1): 8-12
Lorenz K 1971 Studies in Animal and Human Behavior, Volume II. Harvard University Press: Cambridge, USA
Luttrell L, Acker L, Urben M and Reinhardt V 1994 Training a large troop of rhesus macaques to co-operate during catching: analysis of the time investment. Animal Welfare 3: 135-140
Lyons D M, Price E 0 and Moberg G P 1988 Social modulation of pituitary-adrenal responsiveness and individual differences in behavior of young domestic goats. Physiology and Behavior 43:451-458
Mason W A 1991 Effects of social interaction on well-being: development aspects. Laboratory Animal Science 41: 323-328
Maxim P E 1976 An interval scale for studying and quantifying social relations in pairs of rhesus monkeys. Journal of Experimental Psychology 105: 123-147
Mendoza S P 1991 Sociophysiology of well-being in nonhuman primates. Laboratory Animal Science 41: 344-349
Mendoza S P and Mason W A 1986 Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology and Behavior 38: 795-801
Mitchell G and Gomber J 1976 Moving laboratory rhesus monkeys (Macaca mulatta) to unfamiliar home cages. Primates 17: 543-547
National Institutes of Health (NIH) 1985 Guide for the Care and Use of Laboratory Animals. NIH Publication No 85-23: Bethesda, USA
National Institutes of Health (NIH) 1991 Nonhuman Primate Management Plan. Office of Animal Care and Use. NIH: Bethesda, USA
Novak M A and Drewsen K H 1989 Enriching the lives of captive primates: issues and problems. In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 161-182. Noyes Publications: Park Ridge, USA
Novak M A and Suomi S J 1988 Psychological well-being of primates in captivity. American Psychologist 43: 765-773
Novak M A and Suomi S J 1991 Social interaction in nonhuman primates: an underlying theme for primate research. Laboratory Animal Science 41: 308-314
O'Keeffe R T and Lifshitz K 1985 A behavioural profile of stumptailed macaques (Macaca arctoides). Primates 26:143-160
O'Neill P L 1989 A room with a view for captive primates: issues, goals, related research and strategies. In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 135-160. American Psychological Association: Washington, USA
Pereira M E, Macedonia J M, Haring D M and Simson E L 1989 Maintenance of primates in captivity for research: the need for naturalistic environments. In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 40-60. Noyes Publications: Park Ridge, USA
Petto A J 1994 Responses to survey on ethical principles in primatology. Laboratory Primate Newsletter 33(3):3-7
Phillippi-Falkenstein K and Clarke M R 1992 Procedure for training corral-living rhesus monkeys for fecal and blood-sample collection. Laboratory Animal Science 42: 83-85
Post W and Baulu J 1978 Time budgets of Macaca mulatta. Primates 19:125-140
Ranheim S and Reinhardt V 1989 Compatible rhesus monkeys provide long-term stimulation for each other. Laboratory Primate Newsletter 28(3): 1-2
Rasmussen K L R and Reite M 1982 Loss-induced depression in an adult macaque monkey. American Journal of Psychiatry 139: 679-681
Redican W and Mitchell G 1973 The social behavior of adult male-infant pairs of rhesus macaques in a laboratory environment. American Journal of Physical Anthropology 38: 523-526
Reinhardt V 1987 Advantages of housing rhesus monkeys in compatible pairs. Scientists Center for Animal Welfare Newsletter 9(3): 3-6
Reinhardt V 1989 Behavioral responses of unrelated adult male rhesus monkeys familiarized and paired for the purpose of environmental enrichment. American Journal of Primatology 17: 243-248
Reinhardt V 1990a Social enrichment for laboratory primates: a critical review. Laboratory Primate Newsletter 29(3): 7-11
Reinhardt V 1990b Time budget of caged rhesus monkeys exposed to a companion, a PVC perch and a piece of wood for an extended time. American Journal of Primatology 20: 51-56
Reinhardt V 1991a Group formation of previously single-caged adult rhesus macaques for the purpose of environmental enrichment. Journal of Experimental Animal Science 34: 110-115
Reinhardt V 1991b An environmental enrichment program for caged rhesus monkeys at the Wisconsin Regional Primate Research Center. In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 149-159. American Psychological Association: Washington, USA
Reinhardt V 1992a Effective, safe and inexpensive environmental enrichment and improved handling of research rhesus macaques. In: Krulish L (ed) Implementation Strategies for Research Animal Well-Being: Institutional Compliance with Regulations pp 121-126. Scientists Center for Animal Welfare: Bethesda, USA
Reinhardt V 1992b Transport-cage training of caged rhesus macaques. Animal Technology 43: 57-61
Reinhardt V 1992c Improved handling of experimental rhesus monkeys. In: Davis H and Balfour D (eds) The Inevitable Bond. Examining Scientist-Animal Interactions pp 171-177. Cambridge University Press: Cambridge, UK
Reinhardt V 1994a Survey of environmental enrichment for research macaques. Laboratory Primate Newsletter 33(3): 1-2
Reinhardt V 1994b Continuous pair-housing of caged Macaca mulatta: risk evaluation. Laboratory Primate Newsletter 33(1): 1-4
Reinhardt V 1994c Social enrichment for previously single-caged stumptail macaques. Animal Technology 45: 37-41
Reinhardt V 1994d Pair-housing rather than single-housing for laboratory rhesus macaques. Journal of Medical Primatology 23: 426-431
Reinhardt V and Cowley D 1990 Training stumptai led monkeys to cooperate during in-home-cage treatment. Laboratory Primate Newsletter 29(4): 9-10
Reinhardt V and Cowley D 1992 In-homecage blood collection from conscious stumptailed macaques. Animal Welfare 1: 249-255
Reinhardt V, Cowley D and Eisele S 1991 Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. Journal of Experimental Animal Science 34: 73-76
Reinhardt V, Cowley D, Eisele S, Vertein R and Houser D 1988b Pairing compatible female rhesus monkeys for the purpose of cage enrichment has no negative impact on body weight.Laboratory Primate Newsletter 27(1): 13-15
Reinhardt V, Houser D, Cowley D, Eisele S and Vertein R 1989 Alternatives to single caging of rhesus monkeys (Macaca mulatta) used in research. Zeitschrift für Versuchstierkunde 32: 275-279
Reinhardt V, Houser W D, Eisele S G and Champoux M 1987b Social enrichment with infants of the environment for singly caged adult rhesus monkeys. Zoo Biology 5: 365-371
Reinhardt V, Houser W, Eisele S, Cowley D and Vertein R 1988a Behavior responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. American Journal of Primatology 14 135-140
Reinhardt V and Hurwitz S 1993 Evaluation of social enrichment for aged rhesus macaques. Animal Technology 44: 53-57
Reinhardt V and Reinhardt A 1991 Impact of a privacy panel on the behavior of caged female rhesus monkeys living in pairs. Journal of Experimental Animal Science 34: 55-58
Reinhardt V, Reinhardt A, Eisele S, Houser D and Wolf J 1987a Control of excessive aggressive disturbance in a heterogeneous troop of rhesus monkeys. Applied Animal Behaviour Science 18:371-377
Reite M R, Harbeck R and Hoffman A 1981 Altered cellular immune response following peer separation. Life Sciences 29:1133-1136
Rhine R J and Cox R L 1989 How not to enlarge a stable group of stumptailed macaques (Macaca arctoides). In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp 255-269. Noyes Publications: Park Ridge, USA
Rhine R J and Kronwetter C 1972 Interaction patterns of two newly formed groups of stumptailed macaques (Macaca arctoides). Primates 13: 19-33
Rolland R M 1991 A prescription for psychological well-being. In: Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 129-134. American Psychological Association: Washington, USA
Rowell T E and Hinde R A 1963 Responses of rhesus monkeys to mildly stressful situations. Animal Behaviour 11: 235-243
Ruempler U 1992 Beschäftigungsmöglichkeiten bei Primaten im Zoo. Zeitschrift des Kölner Zoo 35(2): 47-68
Ruppenthal G C, Walker C G and Sackett G P 1991 Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages. American Journal of Primatology 25: 103-113
Samuels A and Henrickson R V 1983 Outbreak of severe aggression in captive Macaca mulatta. American Journal of Primatology 5: 277-281
Schapiro S J and Bloomsmith M A 1994 Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. American Journal of Primatology 32: 159-170
Schapiro S J, Bloomsmith M A, Kessel A L and Shively C A 1993 Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science 37:251- 263
Schapiro S J, Bloomsmith M A, Kessel A L, Bushong D and Keeling M E 1991 Effects of environmental enrichment on the social behavior of pair-housed juvenile specific pathogen free (SPF) rhesus monkeys. American Journal of Primatology 24: 133-134 (Abstract)
Schapiro S J and Bushong D 1994 Effects of enrichment on veterinary treatment of laboratory rhesus macaques (Macaca mulatta). Animal Welfare 3: 25-36
Schapiro S J, Lee-Parritz D E, Taylor L L, Watson L, Bloomsmith M A and Petto A 1994 Behavioral management of specific pathogen-free rhesus macaques: group formation, reproduction, and parental competence. Laboratory Animal Science 44: 229-234
Schwind C, Lessnau R G and Taylor L L 1992 An adult male rhesus macaque adopts an orphaned female infant. American Journal of Primatology 27: 57 (Abstract)
Segal E F 1989 Preface. In: Segal E F (ed) Housing, Care and Psychological Well-being of Captive and Laboratory Primates pp vii-xv. Noyes Publications: Park Ridge, USA
Shively C A, Clarkson T B and Kaplan J R 1989 Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis 77: 69-76
Smith E 0 1981 Device for capture and restraint of nonhuman primates. Laboratory Animal Science 31: 305-306
Southwick C H 1967 An experimental study of intragroup agonistic behavior in rhesus monkeys (Macaca mulatta). Behaviour 28: 182-209
Stanton M E, Patterson J M and Levine S 1985 Social influences on conditioned cortisol secretion in the squirrel monkey. Psychoneuroendocrinolgy 10: 125-134
Suomi S J, Eisele C D, Grady S A and Harlow H F 1975 Depressive behavior in adult monkeys following separation from family environment. Journal of Abnormal Psychology 84: 576-578
Taff M A and Doihinow P 1989 Langur monkeys (Presbytis entellus) in captivity. In Novak M A and Petto A J (eds) Through the Looking Glass: Issues of Psychological Well-Being in Captive Nonhuman Primates pp 291-304. American Psychological Association: Washington, USA
Taylor G T 1981 Fear and affiliation in domesticated male rats. Journal of Comparative and Physiological Psychology 95: 685-694
Teas J, Ritchie T, Taylor H and Southwick C 1980 Population patterns and behavioural ecology of rhesus monkeys (Macaca mulatta) in Nepal. In: Lindburg D G (ed) The Macaques pp 247-262. Van Nostrand Reinhold: New York, USA
Tokuda K and Jensen G D 1969 Determinations of dominance hierarchy in a captive group of pigtailed monkeys (Macaca nemestrina). Primates 10: 227-236
US Department of Agriculture 1991 Animal welfare; standards; final rule. Federal Register 56: 6426-6505
Vandenbergh J G 1989. Issues related to "psychological well-being" in nonhuman primates . American Journal of Primatology 1: 9-15
Vertein R and Reinhardt V 1989 Training female rhesus monkeys to cooperate during in-homecage venipuncture. Laboratory Primate Newsletter 28(2): 1-3
Visalberghi E and Anderson J R 1993 Reasons and risks associated with manipulating captive primates' social environments. Animal Welfare 2: 3-15
Washburn D A, Harper S and Rumbaugh D M 1994 Computer-task testing of rhesus monkeys (Macaca mulatta) in the social milieu. Primates 35: 343-351
Whitney R A and Wickings E J 1987 Macaques and other old world simians. In: Poole T B (ed) The UFA W Handbook on the Care & Management of Laboratory Animals, 6th edition pp 599-627. Longman Scientific & Technical: Harlow, UK
Willott J F and McDaniel J 1974 Changes in the behavior of laboratory-reared rhesus monkeys following the threat of separation. Primates 15: 321-326
Wolff A and Ruppert G 1991 A practical assessment of a non-human primate exercise program. Lab Animal 20(2): 36-39
Woolverton W L, Ator N A, Beardsley P M and Carroll M E 1989 Effects of environmental condition on the psychological well-being of primates. A review of the literature. Life Sciences 44:901-917
Published in Animal Welfare 4(4), 307-328 (1995)
© 1995 Universities Federation for Animal Welfare