K Bayne1, S Dexter1, H Mainzer1, C McCully2, G Campbell3 and F Yamada3
1 Office of Animal Care and Use, Office of the Director, National Institutes of Health, 9000 Rockville Pike, Building 14D, Room 313, Bethesda, Maryland 20892, USA
2 Pediatric Branch, National Cancer Institute, National Institutes of Health
3 Division of Computer Research and Technology, National Institutes of Health
Abstract
The provisioning of foraging opportunities to Primates has been shown to be an effective means of enriching the laboratory environment. In this study artificial turf was used as the substrate for a particulate food given to the subjects as an environmental enrichment technique. Eight rhesus monkeys exhibited a significant reduction in behavioural pathology when allowed to extend the amount of time they spent in consummatory activities. An increasing trend in time spent foraging with a concomitant decline in aberrant behaviour over a period of six months was particularly noteworthy. No significant difference in preference for particulate monkey chow or more flavourful particulate food treats was expressed by the primates.
Keywords: animal welfare, environmental enrichment, psychological well-being
Introduction
A precise definition of the term 'psychological well-being' of primates, a term imposed upon the scientific community in a 1985 amendment to the USA Animal Welfare Act, continues to elude experts in the fields of mental health, primatology and veterinary medicine. However, other terms which can be defined, such as 'behavioural well-being' (the manifestation of a behavioural repertoire which approximates that seen in the species under free-ranging conditions) and 'environmental enrichment,' (an environment in which complex stimuli are provided to alleviate the occurrence of abnormal behaviours) are currently being used by investigators attempting to provide research facilities with means of meeting this Congressional mandate (Bayne et al 1991, Bloomsmith & Maple 1988). The overriding implication of these latter terms is that an increase in the complexity of the cage environment, and thus the potential for the animal to engage in normal activities, is behaviourally beneficial to the animal in question. And, indeed, whether the form of enrichment is a social companion (Reinhardt et al 1987b), the ability to perch (Schmidt et al 1989, Wolff 1989), or the opportunity to forage (Anderson & Chamove 1984, Bayne et al 1991, Bloom & Cook 1989, Boccia 1989, Gust et al 1988, McGrew et al 1986), there is an increasing amount of data that support the hypothesis that increased environmental complexity leads to well-being.
A second proposed construct regarding non-human primate well-being is the provision to the animal of some degree of control over its own environment (Novak & Suomi 1988). Control can be accomplished with all three of these major techniques. Control over social opportunities can be allocated to the primate by providing hiding/escape places or, conversely, by allowing the animal to manipulate the cage to increase visual or physical social contacts. The inclusion of a perch in the cage has also been hypothesized as a means of increasing the primate's sense of 'security', as the animal can choose a more elevated position in the cage (Reinhardt et al 1987a).
Foraging opportunities are typically limited in a laboratory situation. The specific nutritional requirements of some non-human primates, such as the rhesus monkey, have been documented (National Research Council 1978) and are adequately met with commercial monkey diets. Also Good Laboratory Practice Regulations (Food and Drug Administration 1978) and Good Laboratory Practice Standards (Environmental Protection Agency 1989) restrict the variability of food items which may be offered to laboratory animals on some studies. Other concerns, such as potential obesity of the animal resulting from the additional calories, means of delivery of the forage, and noninterference with normal laboratory routine, need to be considered. As has recently been demonstrated (Bayne et al 1991), using a forage that is nutritionally balanced and of a small particle size (as a supplement to the twice daily regimen of commercial diet) overcomes many of these concerns. An additional benefit to using particulate forage is the prolongation of time engaged in consummatory activities and the concomitant reduction of abnormal behaviours manifested.
Due to inherent variability in facilities, protocol requirements and individual differences between monkeys, it is clear that no single strategy will have ubiquitous success as an enrichment technique. Consequently, new enrichment methods and modifications of existing methods need to be examined. Accordingly, our laboratory continues to test various substrates for forage which may be placed in the home cage.
Methods
Subjects
Eight adult male rhesus monkeys (Macaca mulatta) were individually housed, although they had social housing experience prior to coming to the facility. They ranged in age from 7 to 10 years. Due to their differences in body- weights (which ranged from 8.0 to 10.54 kg), two animals were housed in 4.3 sq ft (0.4 sq m) cages and six were held in 6.0 sq ft (0.55 sq m) cages to conform with recommendations stated in the Guide for the Care and Use of Laboratory Animals (NH-1 1985). These animals had lived in the same animal-holding room for over a year and were not involved in other research projects at the time of this study. The animals were on a 12L:12D cycle. Purina monkey chow was fed to all animals at 0730 and 1430h. During the study no other additional types of food were given to these animals.
Enrichment apparatus
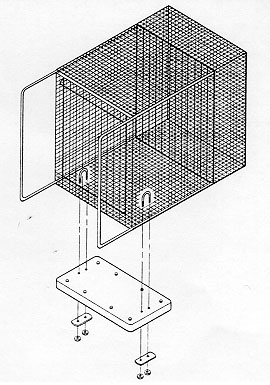
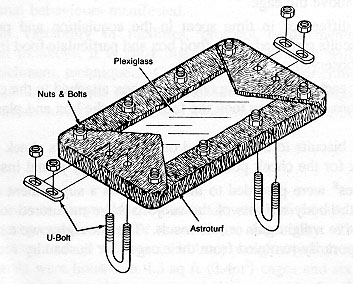
The opportunity for supplemental foraging activities was provided by means of a plexiglass board (30.48 x 58.42 x 0.63cm for the 4.3 sq ft cages and 35.56 x 78.74 x 0.63cm for the 6.0 sq ft cages) covered with artificial turf [polypropylene grass with 'action-back' (burlap), from the 'Proturf series, manufactured by General Felt Industries, Saddlebrook, NJ] with a turf blade length of 1.27cm (Figure 1). The artificial turf was wrapped around and then bolted (Y long, 5/16" - 7.62cm x 0.8cm - stainless steel bolts and nuts) to the plexiglass to prevent it from being destroyed by the primates (it has a burlap backing which rhesus macaques will shred if it is exposed). The board was then placed inside the home cage and secured to the floor of the cage with two U-bolts and wing nuts (thereby preventing any spillage of food material from the board), one on each end of the board (Figure 2). The materials for each board cost approximately US$35. The board occupied approximately one third of the depth of the cage and was situated at the front of the cage. Particles of food material were placed in the artificial turf to stimulate foraging activities. At different times during the study, five flavours were available to the animals: orange, strawberry, cherry, banana, and grape (Crumbles®, Bioserve, Inc). The relative merit of providing monkey chow (NIH Open Formula Extruded Non-human Diet) broken up into particles in the turf in contrast to the more flavourful Crumbles® was also assessed. These small pieces of food would sift down through the blades of turf to varying levels, thereby inducing the animal to engage in different degrees of searching and acquisition behaviours. To minimize risk to personnel, the board was refilled with food particles from outside and above the cage.
To assess the difference in time spent in the acquisition and processing of food between whole biscuits provided in the food box and particulate food in the artificial turf, all subjects were observed on five days for:
- the time it took each animal to empty the food box attached to the cage (this included those cases where the monkey took the food out of the box and placed it on the floor of the cage);
- the time for all biscuits to be consumed or in the monkey's cheek pouch, and
- the time it took for the cheek pouch to appear empty by visual inspection.
As the Crumbles® were provided to the subjects as a supplement to the twice daily feeding regimen, the body-weights of the subjects were monitored to determine if any undesirable excessive weight gain would result. These weights were obtained when the animals were temporarily removed from their cages for husbandry reasons.
The turf board was hosed daily during standard cage cleaning operations. It was not removed from the cage during bi-monthly cage cleaning in a cagewash.
Observational system
A barcode scoring system in conjunction with a laptop computer (Tandy 102) (Line pers comm) was used to record the behaviours of the subjects. All subjects were adapted to the presence of an observer for one week before the initiation of the study. Four 30-min sessions of baseline data were collected for each animal over a two week period, for a total of 16 h (PRE condition). This was followed by twenty 30-min sessions for each subject over six months, for a total of 80 h (EXP condition), two supplementary 30-min sessions per animal to determine if the subjects would forage for small pieces of monkey chow (a food item of presumed lower flavour value than the Crumble®) and another four 30-min sessions with no enrichment in the cage (POST condition). The order of animal observation was randomized and balanced between conditions. Data were collected between 1000 and 1400 h. The turf board was replenished with food particles each day approximately two hours after the morning feeding.
A behaviour manifested by the subjects was considered abnormal if it occurred at a level quantitatively or qualitatively different from that observed in free-ranging populations (after Erwin & Deni 1979). Thus, although grooming is usually considered a normal behaviour, excessive grooming to a degree where the animal is bald in places is considered abnormal. Similarly, cage manipulation or locomotion which occurs in a qualitatively fixed manner, and from which the animal is distracted only with difficulty is considered abnormal. For a list of behaviours which were recorded, see Table 1.
Table 1. Examples of behaviours considered abnormal.
| Repetitive locomotion: | Circle Pace Somersault Rock Spin |
| Stereotypic: | Salute Rub palate with digit Regurgitate Miscellaneous complex idiosyncratic patterns |
| Self-directed: | Self-abuse (self bite, head-banging, etc) Self-clasp Auto-erotic Self-suck Pluck hair Coprophagia Uriposia* Quantitatively abnormal grooming** |
| Cage-directed: | Quantitatively abnormal cage manipulation Cage biting |
|
* Urine dip-stick test was performed on subjects exhibiting this behavior to ensure that glucosuria was not the cause of urine drinking. All subjects tested negative. |
|
Statistical analysis
For purposes of statistical analysis, the wide variety of abnormal behaviours exhibited by the subjects was considered in four main categories: locomotion, self-directed, cage-directed, and stereotypic (Tables 2a and 2b).
Initially, the sum of all four categories was analyzed. The test statistic used for this overall analysis to compare EXP and PRE and POST conditions is a sum over monkeys of ordered non-parametric tests of Jonckheere in a randomized block design (Hollander & Wolfe 1972). Only if there is evidence to support the contention that there are overall differences in the sum of the abnormal behaviours (frequency or duration), are the individual behaviours examined separately (locomotion, stereotypic, self-directed and cage-directed). See the appendix of Bayne et al (1991) for further discussion of this statistical analysis. Page's test is used to test for monotone change over time for total duration of abnormal behaviours and of foraging in the experimental phase (Hollander & Wolfe 1972).
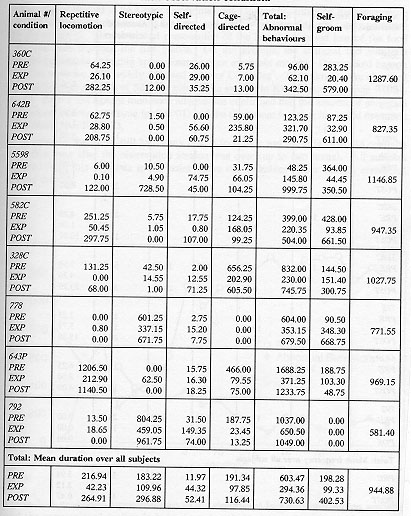
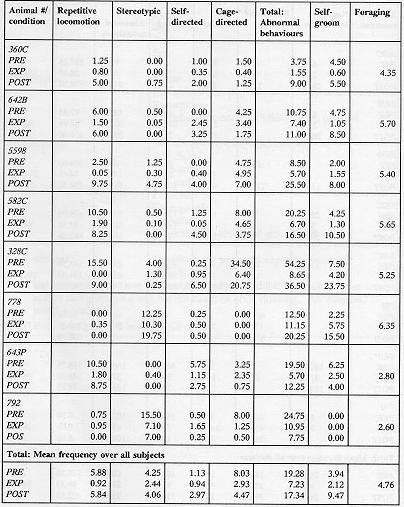
Table 2a & 2b. Mean duration (sec)/30-min session of recorded behaviour
for each observation condition.
Results
Artificial turf proved to be a very sturdy material. It readily withstood the temperature of the cage-wash, routine washing by hose, and daily wear (standing and sitting postures) by the primates. The horizontal presentation of the board prevented loss of the food particles from between the turf blades. On the average, the artificial turf substrate required complete changing approximately once every six months. Orange and banana flavours were the most widely accepted types of Crumble® . The animals were observed to forage for an average of 52.2 per cent of the session in EXP treatment (range = 2.2475%). Page's test against monotone alternatives confirmed that the amount of abnormal behaviour declines over time (p < 0.0001) and the time spent foraging increased over time (p < 0.0001), see Figure 3.
Frequently the board still had many bits of food in it and the animal would continue to forage long after the observation session was over (up to two hours). All subjects foraged from this substrate to the point where the Crumble® were entirely consumed during the day.
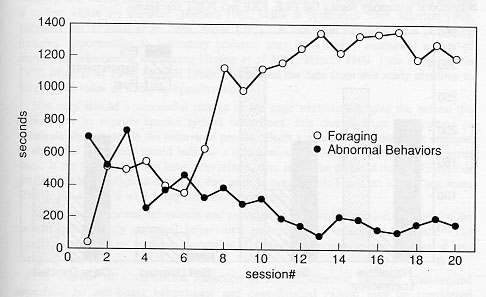
Bouts of self-grooming were occasionally induced when the animals' hands had become moist from licking the particles off the fingers. No significant difference was noted between the time spent foraging for Crumbles' and the time spent foraging for monkey chow particles.
The animals displayed a minimum of four different types of aberrant behaviour during the initial baseline sessions, which did not necessarily include behaviours from all categories of abnormal behaviour (as noted in Tables 2a and 2b). The overall analysis of the total of the four categories of behaviours demonstrated that the EXP condition duration and frequency of these behaviours were significantly lower than the PRE condition (p < 0.002, p < 0.0001) and POST (p < 0.0001, p < 0.0001) with no evidence for a difference between the PRE and POST conditions. Specifically, the repetitive locomotion category was significantly reduced in the EXP condition as compared to the PRE condition (duration: p < 0.0004, frequency: p < 0.0001) and POST condition (duration: p < 0.0004, frequency: p < 0.0004). Self-directed behaviour was higher in duration and frequency in the POST condition than the EXP condition (p < 0.003, p < 0.005). Also, the duration of stereotypic behaviour was significantly reduced when a foraging opportunity was available in the EXP condition (p < 0.05). There was no evidence to support that cage-directed behaviour in the EXP condition was lower in either duration or frequency than in the PRE or POST conditions. Figure 4 presents the relative differences in behavioural pathology during the PRE, EXP and POST conditions.
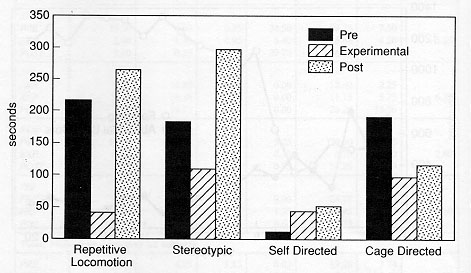
A separate analysis for self-grooming behaviour revealed that the PRE, EXP and POST conditions differed in duration and frequency at p < 0.0001. In particular, the EXP condition had significantly lower durations and frequencies than the PRE condition (p=0.022, p < 0.02) and the POST condition (p < 0.0001, p < 0.0001).
On the average, over the course of a year (this includes the PRE, EXP and POST conditions) the animals gained 0.92kg. No noticeable reduction in biscuit consumption was noted by the animal care staff.
The biscuit consumption survey found the mean time for these subjects to empty the food box of monkey chow biscuits was 5 min. On the average, the subjects had at a minimum pouched (+/- eaten) the biscuits in 13 min, and had what appeared to be empty pouches in approximately 47 min (or 18% of the light cycle).
Discussion
Early on in the consideration of how best to provide for an enriched environment, it was proposed that 'species appropriate' activities be encouraged because one salient approach to improving the well-being of an animal is providing the opportunity for the animal to express the same types of behaviour it would in nature (Line 1987). Evidence that foraging is a varied and time consuming activity in which free-ranging primates engage is replete within the literature (Herbers 1981, Malik & Southwick 1988, Marriott 1988, Milton 1980, O'Neill et al 1989, Strier 1987). It has been suggested that providing foraging opportunities to laboratory primates may be a highly successful means of increasing behavioural well-being (Bayne et al 1991, Boccia 1989, Line & Houghton 1987, Maki et al 1989, O'Neill 1988), and indeed the data from this study continue to support the value of foraging opportunities.
Not only should a successful change to the cage environment give the animal the opportunity to express species typical behaviours, this change must also reduce or eliminate aberrancies in the behaviour profile. Such a reduction in time spent engaged in abnormal behaviour would indicate a substitution of normal activities for abnormal ones. If the substitution has long-ten-n effects, then it may be deduced that the behaviour profile has been adjusted closer to that seen in nature and, based on Line's argument (1987), the animal's well-being has been improved.
Stereotypic abnormal behaviours and excessive repetitive locomotion are perhaps the two most frequently observed behavioural pathologies in laboratory primates. The significant reductions in these categories of behaviour underscore the success of this foraging substrate.
The absence of a significant reduction in the self-directed category (demarcated especially by self-biting behaviours) and cage-directed (which may be considered exploratory behaviour) may have been due to the low frequency of these types of behaviours by this particular subject pool. However, it appears that these behaviours may be more resistant to environmental manipulation.
Although the foraging activities performed by some animals resulted in bouts of self-grooming, the overall level of self- directed grooming was lower when the turf board was present. This overall reduction may have been due to a substitution of what could be classified as an abnormally high level of self-grooming for another behaviour - in this case, foraging. Indeed, the hair coat of some subjects, which had been groomed to the point of baldness, improved during the course of this study. Periodic subjective evaluations of the subjects' behaviours several hours after presentation of the foraging materials revealed long passive periods (resting, visual scanning, grooming) occasionally punctuated with that subject's pattern of behavioural aberrancy.
The brief examination of monkey chow biscuit consumption by these subjects revealed that approximately 18 per cent of the light cycle was spent in eating and processing this food. This value is skewed toward the lower end of the range of time free-ranging primates spend in foraging-related activities (7-65%). The amount of time spent in consummatory behaviour was inflated to a mean of approximately 52 per cent when particulate food was made available to the animals. This latter value, although falling into the higher end of the typical foraging range of time, did not result in any untoward weight gain by the subjects. An analysis of weight gained by individual animals relative to the animal's abnormal behaviour profile did not demonstrate any relationship between a reduction in repetitive locomotion and degree of weight gain. While non-human primates could be fed entirely from foraging substrates, the logistical feasibility of this is difficult. A very large surface area would be required to provide the primate with enough particulate food to spread an equivalent caloric and volume equivalent of food on the board. This is neither a practical nor economic solution to enhancing wen-being.
From a husbandry perspective, the fact that these animals exhibited no difference in foraging time between flavourful food treats and particulate biscuits suggests that waste of biscuits can be reduced. Specifically, the particulate matter at the bottom of the container holding monkey chow need not be disposed of, but rather can be used to supplement the twice daily ration of biscuits. Also, since the particulate food may be sprinkled onto the turf board from above and outside the cage, no additional risk to facility personnel is likely to be generated from this enrichment device.
The use of artificial turf as a foraging substrate made this enrichment device in many ways easier to manage than the fleece substrate described by Bayne et al (1991). Although both substrates resulted in significant reductions in abnormal behaviour, and with both devices the subjects showed increased usage of the enrichment technique (a mean use over time of approximately 52% for artificial turf and 40% for fleece), the artificial turf proved to be quicker to re-load with particulate food (as it did not have to be detached from the cage first). It also was not necessary to remove the board from the cage for cleaning purposes (as was the fleece board). However, unlike the fleece board, no grooming activities directed at the board were noted and no social signals were directed to the turf board (see Bayne et al 1991). One possible disadvantage to the artificial turf substrate is related to its positioning on or under the cage floor. Occasionally, the animals did defecate on the turf. Some would later toss the faeces out of the cage, and others would ignore it. None of the animals observed foraged close to an area where faeces were deposited. The faecal matter was hosed off the board with the daily cleaning. As was stated earlier, no single device will provide the complete solution to well-being. Thus, variations on a successful theme will give scientists, veterinarians, and facility managers more enrichment options from which to choose to meet their particular set of management requirements.
Conclusions
1. The use of artificial turf as a substrate for foraging material proved to be successful in terms of reducing some key abnormal behaviours and non-interference with facility husbandry routines.
2. The amount of time spent foraging by the primates was independent of the two types of foraging materials provided.
3. The artificial turf foraging board does not compromise personnel safety.
Acknowledgements
The authors would like to thank the Pediatric Branch, Leukemia Biology Section, National Cancer Institute, for the use of their monkeys during the course of the study and assistance in data collection by Anne Hicks, Jennifer Kenworthy, Marianna Schafer and Kelly Stockton.
References
Anderson J R, Chamove A S 1984 Allowing captive primates to forage, pp 253-256. In Standards in Laboratory Animal Management. Universities Federation for Animal Welfare: Potters Bar
Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F, Suomi S 1991 The reduction of abnormal behaviors in individually housed rheses monkeys (Macaca mulatta) with a foraging/grooming board. American Journal of Primatology 23: 23-33
Bloom K R, Cook M 1989 Environmental enrichment: behavioral responses of rhesus to puzzle feeders. Lab Animal 18(5): 23-31
Bloomsmith M, Maple T 1988 Enrichment strategies and techniques currently in use. Workshop on Chimpanzee Behavior. National Institutes of Health: Bethesda MD (USA)
Boccia M L 1989 Preliminary report on the use of a natural foraging task to reduce aggression and stereotypies in socially housed pigtail macaques. Laboratory Primate Newsletter 28: 3-4
Environmental Protection Agency 1989 Good Laboratory Practice Standards. Title 40, Code of Federal Regulations, Part 792
Erwin J, Deni R 1979 Strangers in a strange land; abnormal behaviors or abnormal environments? In Erwin J, Maple T, Mitchell G (eds) Captivity and Behavior, pp 128. Van Nostrand Reinhold Co: New York
Food and Drug Administration 1978 Good Laboratory Practice Regulations. Title 21, Code of Federal Regulations Part 58
Gust D A, Swenson K B, Smith M B, Sikes J 1988 An apparatus and procedure for studying the choice component of foraging in a captive group of chimpanzees. Primates 29(1: 139-143
Herbers J M 1981 Time resources and laziness in animals. Oecologia 49(2): 252-262
Hollander M, Wolfe D A 1972 Nonparametric Statistical Methods. John Wiley and Sons: New York
Line S W 1987 Environmental enrichment for laboratory primates. Journal of the American Veterinary Medical Association 190: 854-859
Line S W, Houghton P 1987 Influence of an environmental enrichment device on general behavior and appetite in rhesus macaques. Laboratory Animal Science 37: 508
Maki S, Alford P, Bloomsmith M, Franklin J 1989 A food puzzle device simulating termite-fishing for captive chimpanzees (Pan troglodytes). American Journal of Primatology Supplement 1: 71-78
Malik 1, Southwick C. H 1988 Feeding behavior and activity patterns of rhesus monkeys (Macaca mulatta) at Tughlaqabad, India. In Fa J E, Southwick C H (eds) Ecology and Behavior of Food- Enhanced Primate Groups, pp 95-111. Alan R Liss: New York
Marriott B M 1988 Time budgets of rhesus monkeys (Macaca mulatta) in a forest habitat in Nepal and on Cayo Santiago. In Fa J E, Southwick C H (eds) Ecology and Behavior of Food-Enhanced Primate Groups, pp 125-149. Alan R Liss: New York
McGrew W C, Brennan J A, Russell J 1986 An artificial 'gum-tree' for marmosets (Callithrix j. jacchus). Zoo Biology 5: 45-50
Milton K 1980 The Foraging Strategies of Howler Monkeys: A Study in Primate Economics. Columbia University Press: New York
National Research Council 1978 Committee on Animal Nutrition, Panel on Nonhuman Primate Nutrition. Board on Agriculture and Renewable Resources, Nutrient Requirements of Nonhuman Primates. Nutrient Requirements of Domestic Animals. Vol 14. National Academy of Sciences Press: Washington D C
NIH 1985 Guide for the Care and Use of Laboratory Animals. National Institutes of Health: Bethesda, MD, (USA)
Novak M, Suomi S 1988 Psychological well-being of primates in captivity. American Psychologist 43: 765- 773
O'Neill P 1988 Developing effective social and environment enrichment strategies for macaques in captive groups. Lab Animal 17: 23-36
O'Neill P, Price C, Suomi S 1989 Daily patterns in activity levels relative to age and sex in a free-ranging group of rhesus monkeys. American Association of Laboratory Animal Scientists Meeting. Little Rock, Arkansas
Reinhardt V, Houser W D, Cowley D, Champoux M 1987a Preliminary comments on environmental enrichment with branches for individually caged rhesus monkeys. Laboratory Primate Newsletter 26(1): 1-3
Reinhardt V, Houser W D, Eisele S G, Champoux M 1987b Social enrichment of the environment with infants for singly caged adult rhesus monkeys. Zoo Biology 6: 365-371
Schmidt E M, Dold G M, McIntosh J S 1989 A perch for primate squeeze cages. Laboratory Animal Science 39: 166-167
Strier K B 1987 Activity budgets of woolly spider monkeys, or muriquis (Brachyteles arachnoides). American Journal of Primatology 13: 385-395
Wolff A V 1989 Polyvinyl chloride piping as perch material for squirrel monkeys. Laboratory Primate Newsletter 22(1): 7
This article originally appeared in Animal Welfare 1: 39-53 (1992)
Reprinted with permission of the publisher.