Viktor Reinhardt
Animal Welfare Institute
Washington, D.C.
Training macaques to cooperate during blood collection is a practicable and safe alternative to the traditional procedure implying forced restraint. It takes a cumulative total of about 1 hr to train an adult female or adult male rhesus macaque successfully to present a leg voluntarily and accept venipuncture in the homecage. Cooperative animals do not show the significant cortisol response and defensive reactions that typically occur in animals who are forcibly restrained during this common procedure.
Blood collection is probably the most common handling procedure nonhuman primates are subjected to in research institutions. Traditionally, it is accomplished by forciblyrestraining the subject because it is believed that "all monkeys are dangerous" (Ackerley & Stones, 1969, p. 207), that "nonhuman primates can be very difficult and even dangerous to handle," and that "restraint is therefore necessary and desirable to protect both the investigator and the animal" (Robbins, Zwick, Leedy, & Stearns, 1986, p. 68). Indeed, a subdued monkey will try to show self-defensive aggression. Therefore, "despite rigorous observance of all precautions, bites and scratches are frequent" (Valerio et al., 1969, p. 45; Zakaria, Lerche, Chomel, & Kass, 1996).
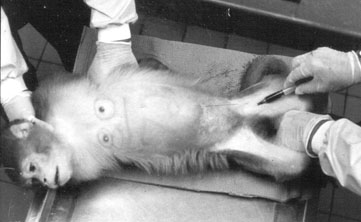
THE VARIABLE
Because of "adverse conditioning or fear" (Robbins, Zwick, Leedy, & Stearns, 1986, p. 68) enforced restraint during blood collection is an extremely alarming situation (see Figure 1) that affects physiological equilibrium, thereby increasing data variability and the number of research subjects needed to achieve statistically significant results (Brockway, Hassler, & Hicks, 1993). It has been shown that compulsory restraint changes normative:
- Cortisol secretion in rhesus (Elvidge, Challis, Robinson, Roper, & Thorburn, 1976; Fuller, Hobson, Reyes, Winter, & Faiman, 1984; Hayashi & Moberg, 1987; Line, Markowitz, Morgan, & Strong, 1991; Puri, Puri, & Anand-Kumar, 1981) and Japanese macaques (Torii, Kitagawa, Nigi, & Ohsawa, 1993) as well as in capuchin monkeys (Dettmer, Phillips, Rager, Bernstein, & Fragaszy, 1996);
- Progesterone secretion in baboons (Albrecht, Nightingale, & Townsley, 1978; Goncharov et al., 1979);
- Testosterone secretion in rhesus macaques (Hayashi &Moberg, 1987; Puri, Puri, & Anand-Kumar, 1981) and baboons (Goncharov et al., 1979);
- Adrenal androgen secretion in rhesus macaques (Fuller, Hobson, Reyes, Winter, & Faiman, 1984);
- Prolactin secretion in rhesus macaques (Quadri, Pierson, & Spies, 1978);
- Growth hormone secretion in rhesus macaques (Mason et al., 1968);
- Follicle stimulating hormone secretion in rhesus macaques (Todd et al., 1999);
- Glucagon secretion in squirrel monkeys (Myers, Mendoza, & Cornelius, 1988);
- Glucose regulation in rhesus, stump-tailed (Streett & Jonas, 1982) and Celebes macaques (Yasuda, Wolff, & Howard, 1988);
- Serum glutamic-oxalacetic transaminase activity in rhesus macaques (Cope & Polis, 1959);
- Aspartate aminotransferase and alanine aminotransferase activity in long-tailed macaques (Landi, Kissinger, Campbell, Kenney, & Jenkins, 1990);
- White blood cell count in rhesus macaques (Ives & Dack, 1956; Loomis, Henrickson, & Anderson, 1980) and baboons (Goosen, Davies, Maree, & Dormehl, 1984);
- Blood concentration in rhesus macaques (Loomis, Henrickson, & Anderson, 1980);
- Blood pressure and heart rate in rhesus macaques (Golub & Anderson, 1986) and marmosets (Schnell & Wood, 1993);
- Acid-base balance in squirrel monkeys (Manning, Lehner, Feldner, &Bullock, 1969) and Barbary and lion-tailed macaques (Bush, Custer, Smeller, & Bush, 1977); and
- Respiration rate in rhesus and long-tailed macaques (Berendt & Williams, 1971).
Surprisingly, traditional blood sampling is officially "expected to produce little or no discomfort" (Scientists Center for Animal Welfare, 1987, p. 12). In line with this, many investigators tacitly ignore their subjects' stress responses during blood collection (Reinhardt & Reinhardt, 2000).
THE REFINEMENT
In an attempt to reduce the stress reaction during blood collection, six individually caged adult (8 to 12 years old) female rhesus macaques (Macaca mulatta) were trained to cooperate during femoral venipuncture in the homecage (see Figure 2). The subjects were used to being immobilized on a table for this procedure (see Figure 1).
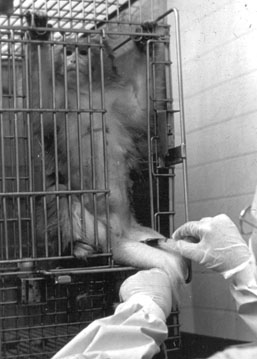
The effect of the training was assessed by drawing two blood samplesthe first at 13:15 ±1 min and the second at 13:30 ±1 minfrom each animal during the conventional procedure involving forced restraint and, on another day, during the refined procedure involving voluntary cooperation. The animals lived in 70 cm × 75 cm × 77 cm large upper row cages. They were fed commercial dry food at 7:30 and fruit at 15:00. The macaques were subjected to no external disturbanceincluding personnel walking in the hallwaysfor 1.5 hr before the first blood drawing at 13:15. Both during the conventional and during the refined procedure, venipuncture occurred 60 to 90 sec after the caretaker had entered the animal room. The time lapse did not differ between the two conditions (conventional 76 ± 12 sec vs. refined 73 ± 14 sec; t = 0.399, df = 5, p > .1).
The blood samples were analyzed for serum cortisol as an indicator of stress. The first samples were used to assess basal levels; the second samples served to evaluate the magnitude of the cortisol response 15 min after venipuncture. Mean cortisol concentrations of the first samples did not differ under both conditions, t = 0.226, df =5,p > .1 (Table 1). Cortisol concentrations of the second samples, however, were significantly higher under the restraint condition than under the cooperation condition, t = 3.910, df =5,p < .005 (Table 1). The magnitude of the endocrine response to venipuncture was significant (+68%), t = 4.834, df = 5, p < .001, when the subjects were restrained, but it was insignificant (+14%), t = 1.135, df = 5, p > .1, when they cooperated (see Table 1).
THE TRAINING PROTOCOL
The following protocol was used to train the subjects of this study as well as 12 adult pair-housed female, 5 adult single-housed male, 10 adult pair-housed male rhesus macaques, and 6 adult pair-housed female stump-tailed macaques (M. arctoides). The animals were used to being immobilized mechanically in their homecages during routine procedures such as ketamine injection.
Step 1
Establish an affectionate relationship with the trainee. She or he should come to the front of the cagerather than retreat to the backwhen you enter the room. The subject must trust you; only then will it be safe to proceed with the training.
Step 2
With the help of the squeeze-back, the subject is confined in the front quarter of the cage. In this position, freedom of movement is considerably restricted, but the subject has enough leeway to turn around. The animal is reassuringly talked to, gently scratched through the mesh, and offered some raisins. After a minute or two, the squeeze-back is pushed back and raisins again are offered. This exercise is repeated on different days until the animal is relaxed and accepts the food reward.
Step 3
The subject again is restricted and enticed with raisins and/or gently prodded to face the left or right side of the cage. The subject's leg is touched and groomed through the opening of the door. After a minute or two, the squeeze-back is pushed back and raisins offered. This sequence of events is repeated on different days until the animal stops retracting the leg and accepts the food reward.
Step 4
The restricted subject's leg is gently and firmly pulled through the opening of the door and held firmly for about 1 minute. The squeeze-back is pushed back and the subject rewarded with raisins. The goal of Step 4 is achieved when the animal shows no signs of resistance such a trying to retract the leg or to turn around.
TABLE 1
Cortisol Responses of Six Rhesus Macaques to Traditional and Refined Blood Collection
| Blood Sampling Procedure | Mean Cortisol Concentrations | Difference (Significance) | |
|---|---|---|---|
| First Sample | Second Sample | ||
| Traditional (restraint) | 20.1 ± 4.5 µg/dl | 33.8 ± 5.3 µg/dl | p < .001 |
| Refined (cooperation) | 19.6 ± 3.0 µg/dl | 22.3 ± 5.0 µg/dl | p < .1 |
Step 5
The squeeze-back is pulled only so far as to prompt the trainee to come forward. The animal is in full control of the situation and has enough room to turn around freely and avoid being touched. The trainer encouragingly asks the subject to present a leg behind or through the opening of the door. An animal who refuses to cooperate is not punished in any manner but simply does not receive a food reward. This exercise is repeated on different days until the animal actively presents a leg and shows no resistance during blood collection from the femoral vein (see Figure 2) or saphenous vein. Once this goal is achieved, the animal is praised and rewarded with raisins.
The training protocol outlined here was applied successfully not only by the author but also by two animal caretakers, Vertein (Vertein & Reinhardt, 1989) and Cowley (Reinhardt & Cowley, 1992).
THE TIME INVESTMENT
The total number of training sessions per animal ranged from 2 to 27. Individual training sessions lasted from a few seconds to 5 minutes, depending on the trainee's responsiveness. Cumulative time to reach the training goal (Step 5) ranged from 16 to 69 min with a mean of 38.5 min (see Table 2). There was a tendency, statistically insignificant, for pair-housed subjects requiring less training time than single-housed subjects; female rhesus: t = 0.621, df = 16, p > .1; male rhesus: t = 0.469, df = 13, p > .1 (Table 2). Females and males did not differ in the time needed to train them; rhesus pair-housed: t = 0.025, df = 20, p >.1; rhesus single-housed: t = 0.065, df = 9, p > .1 (see Table 2).
Although traditional blood sampling procedures usually require at least two peopleone to help restrain the subject, one to puncture a vein and draw blood (see Figure 1)only one person is required to do this procedure with a trained subject (see Figure 2). Once trained, all animals cooperated not only with the trainer but also with the attending care personnel as well as with experienced personnel from other facilities.
TABLE 2
Time Investment to Achieve Active Cooperation of Macaques
During Blood Collection in the Familiar Homecage
| Subject, Housing | n | Time Investment (Minutes) |
|---|---|---|
| Female rhesus, single-housed | 6 | 44.3 ± 16.6 |
| Female rhesus, pair-housed | 12 | 39.0 ±18.0 |
| Male rhesus, single-housed | 5 | 43.6 ± 18.7a |
| Male rhesus, pair-housed | 10 | 38.8 ± 18.6a |
| Female stump-tailed, pair-housed | 6 | 33.5 ± 10.0b |
a Data originally published in Reinhardt (1991). b Data originally published in Reinhardt and Cowley (1992).
CONCLUSIONS
Training macaques to cooperate voluntarily during blood collection is a practical alternative to the traditional procedure implying forced restraint and stress. Working with a cooperative rather than against a resisting monkey (a) eliminates the handler's risk of becoming the target of defensive biting and scratching; (b) refines research methodology by controlling the extraneous variable of stress; and (c) provides high quality mental stimulation both to the animal and to the handling person. The initial time investment in the training quickly pays off in a safe handling procedure that no longer requires a second person to control the resisting subject. It should be noted that the idea of training macaques to cooperate during blood collection is not new. There are reports from 10 different research facilities where macaques have been trained voluntarily to present a leg for blood collection (Reinhardt, 1997). Surprisingly, however, this simple, yet effective, refinement technique is applied only sporadically while the traditional technique relying on force still is prevailing.
ACKNOWLEDGMENT
This project was partly supported by NIH Grant RR00167 to the Wisconsin Regional Primate Research Center.
REFERENCES
Ackerley, E. T., & Stones, P. B. (1969). Safety procedures for handling monkeys. Laboratory Animal Handbooks, 4, 207211.
Albrecht, E. D., Nightingale, M. S., & Townsley, J. D. (1978). Stress-induced decrease in the serum concentration of progesterone in the pregnant baboon. Journal of Endocrinology, 77, 425426.
Berendt, R., & Williams, T. D. (1971). The effect of restraint and position upon selected respiratory parameters of two species of Macaca. Laboratory Animal Science, 21, 502509.
Brockway, B. P., Hassler, C. R., & Hicks, N. (1993). Minimizing stress during physiological monitoring. In S. M. Niemi, & J. E. Willson (Eds.), Refinement and reduction in animal testing (pp. 5669) Bethesda, MD: Scientists Center for Animal Welfare.
Bush, M., Custer, R., Smeller, J., & Bush, L. M. (1977). Physiologic measures of nonhuman primates during physical restraint and chemical immobilization. Journal of the American Veterinary Medicine Association, 171, 866869.
Cope, F. W., & Polis, B. D. (1959). Increased plasma glutamic-oxalacetic transaminase activity in monkeys due to nonspecific stress effect. Journal of Aviation Medicine, 30, 9094.
Dettmer, E. L., Phillips, K. A., Rager, D. R., Bernstein, I., & Fragaszy, D. M. (1996). Behavioral and cortisol responses to repeated capture and venipuncture in Cebus apella. American Journal of Primatology, 38, 357362.
Elvidge, H., Challis, J. R. G., Robinson, J. S., Roper, C., & Thorburn, G. D. (1976). Influence of handling and sedation on plasma cortisol in rhesus monkeys (Macaca mulatta). Journal of Endocrinology, 70, 325326.
Fuller, G. B., Hobson, W. C., Reyes, F. I., Winter, J. S. D., & Faiman, C. (1984). Influence of restraint and ketamine anesthesia on adrenal steroids, progesterone, and gonadotropins in rhesus monkeys. Proceedings of the Society for Experimental Biology and Medicine, 175, 487490.
Golub, M. S., & Anderson, J. H. (1986). Adaptation of pregnant rhesus monkeys to short-term chair restraint. Laboratory Animal Science, 36, 507511.
Goncharov, N. P., Taranov, A. G., Antonichev, A. V., Gorlushkin, V. M., Aso, T., Ckan, S. Z., et al. (1979). Effects of stress on the profile of plasma steroids in baboons (Papio hamadryas). Acta Endocrinologica, 90, 372384.
Goosen, D. J., Davies, J. H., Maree, M., & Dormehl, I. C. (1984). The influence of physical and chemical restraint on the physiology of the chacma baboon (Papio ursinus). Journal of Medical Primatology, 13, 339351.
Hayashi, K. T., & Moberg, G. P. (1987). Influence of acute stress and the adrenal axis on regulation of LH and testosterone in the male rhesus monkey (Macaca mulatta). American Journal of Primatology, 12, 263273.
Ives, M., &Dack, G. M. (1956). "Alarm reaction" and normal blood picture in Macaca mulatta. Journal of Laboratory Clinical Medicine, 47, 723729.
Landi, M. S., Kissinger, J. T., Campbell, S. A., Kenney, C. A., & Jenkins, E. L. (1990). The effects of four types of restraint on serum alanine aminotransferase and asparate aminotransferase in the Macaca fascicularis. Journal of the American College of Toxicology, 9, 517523.
Line, S. W., Markowitz, H., Morgan, K. N., & Strong, S. (1991). Effect of cage size and environmental enrichment on behavioral and physiological responses of rhesus macaques to the stress of daily events. In M. A. Novak & A. J. Petto (Eds.), Through the looking glass: Issues of psychological well-being in captive nonhuman primates (pp. 160179) Washington, DC: American Psychological Association.
Loomis, M. R., Henrickson, R. V., & Anderson, J. H. (1980). Effects of ketamine hydrochloride on the hemogram of rhesus monkeys (Macaca mulatta). Laboratory Animal Science, 30, 851853.
Manning, P. J., Lehner, N. D. M., Feldner, M. A., & Bullock, B. C. (1969). Selected hematologic, serum chemical, and arterial blood gas characteristics of squirrel monkeys (Saimiri sciureus). Laboratory Animal Care, 19, 831837.
Mason, J. W., Wool, M. S., Wherry, F. E., Pennington, L. L., Brady, J. V., & Beer, B. (1968). Plasma growth hormone response to avoidance in the monkey. Psychosomatic Medicine, 30, 760773.
Myers, B. A., Mendoza, S. P., & Cornelius, C. E. (1988). Elevation of plasma glucagon levels in response to stress in squirrel monkeys: Comparison of two subspecies (Saimiri sciureus boliviensis and Saimiri sciureus sciureus). Journal of Medical Primatology, 17, 205214.
Puri, C. P., Puri, V., & Anand-Kumar, T. C. (1981). Serum levels of testosterone, cortisol, prolactin and bioactive luteinizing hormone in adult male rhesus monkeys following cage-restraint or anaesthetizing with ketamine hydrochloride. Acta Endocrinologica, 97, 118124.
Quadri, S. K., Pierson, C., & Spies, H. P. (1978). Effects of centrally acting drugs on serum levels in rhesus monkeys. Neuroendocrinology, 27, 136147.
Reinhardt, V. (1991). Training adult male rhesus monkeys to actively cooperate during in-homecage venipuncture. Animal Technology, 42, 1117.
Reinhardt, V. (1997). Training nonhuman primates to cooperate during blood collection: A review. Laboratory Primate Newsletter, 36(4), 14.
Reinhardt, V., & Cowley, D. (1992). In-homecage blood collection from conscious stumptailed macaques. Animal Welfare, 1, 249255.
Reinhardt, V., & Reinhardt, A. (2000). Blood collection procedure of laboratory primates: A neglected variable in biomedical research. Journal of Applied Animal Welfare Science, 3, 321333.
Robbins, D. Q., Zwick, H., Leedy, M., & Stearns, G. (1986). Acute restraint device for rhesus monkeys. Laboratory Animal Science, 36, 6870.
Schnell, C. R., & Wood, J. M. (1993). Measurement of blood pressure, heart rate, body temperature, ECG and activity by telemetry in conscious unrestrained marmosets.Proceedings of the Fifth Federation of European Laboratory Animal Science Associations Symposium, 107111.
Scientists Center for Animal Welfare. (1987). Consensus recommendations on effective institutional animal care and use committees. Laboratory Animal Science, 37, 1113.
Streett, J. W., & Jonas, A. M. (1982). Differential effects of chemical and physical restraint on carbohydrate tolerance testing in nonhuman primates. Laboratory Animal Science, 32, 263266.
Todd, H. E., Shideler, S. E., Laughlin, L. S., Overstreet, J. W., Pohl, C. R., Byrd, W., et al. (1999). Application of an enzyme immunoassay for urinary follicle-stimulating hormone to describe the effects of an acute stressor at different stages of the menstrual cycle in female laboratory macaques. American Journal of Primatology, 48, 135151.
Torii, R., Kitagawa, N., Nigi, H., & Ohsawa, N. (1993). Effects of repeated restraint stress at 30-minute intervals during 24-hours on serum testosterone, LH and glucocorticoids levels in male Japanese monkeys (Macaca fuscata). Experimental Animal, 42, 6773.
Valerio, D. A., Miller, R. L., Innes, J. R. M., Courntey, K. D., Pallotta, A. J., & Guttmacher, R. M. (1969). Macaca mulatta. Management of a laboratory breeding colony. New York: Academic.
Vertein, R., & Reinhardt, V. (1989). Training female rhesus monkeys to cooperate during in-homecage venipuncture. Laboratory Primate Newsletter, 28(2), 13.
Yasuda, M., Wolff, J., & Howard, C. F. (1988). Effects of physical and chemical restraint on intravenous glucose tolerance test in crested black macaques (Macaca nigra). American Journal of Primatology, 15, 171180.
Zakaria, M., Lerche, N. W., Chomel, B. B., & Kass, P. H. (1996). Accidental injuries associated with nonhuman primate exposure at two Regional Primate Research Centers (U.S.A.): 19881993. Laboratory Animal Science, 46, 298304.
Reproduced with permission of Lawrence Erlbaum Associates
from Journal of Applied Animal Welfare Science 6 (3), 189-197, 2003