By Viktor Reinhardt
Editors' introduction
Like Boccia et al., Reinhardt works with a population of captive primates from which blood samples must be obtained. However, Reinhardt's chapter stands in marked contrast to that of Boccia et al. Both Reinhardt and Boccia et al. recognize the importance of the scientist- animal bond, but deal with it in different ways. Whereas Boccia et al. try to avoid the effects of the bond, Reinhardt attempts to exploit its benefits in order to expedite data collection and minimize handling-associated excitation.
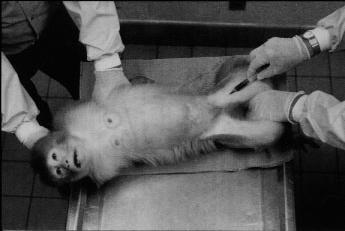
When physical strength, heavy leather gloves, and/or mechanical squeeze devices are used to control experimental rhesus monkeys, the handling is associated with considerable commotion. Flight attempts, threatening behavior, overt aggressive defense behavior, struggling, crouching, fear grinning, and psychogenic diarrhea are common reactions to such procedures and are indicative of the restrained animals' intense aversion (Figure 10.1). Handling-associated excitation, furthermore, is likely to affect the basal physiology of the research subject and hence the validity of the research data collected on it.
In an attempt to minimize handling-associated excitation and thereby to enhance the quality of research conducted on rhesus monkeys, the following techniques were developed at the Wisconsin Regional Primate Research Center:
1. Venipuncture in the home cage. Research subjects are transferred to squeeze-back cages. After an adequate period of familiarization, they become used to having their cage space periodically reduced by about 75%. In this situation, they are trained to permit the caretaker to touch and groom them and finally to show no resistance to having a leg gently pulled out of the partially opened cage door for venipuncture (Figure 10.2). To avoid panic reactions, the experimental animal is not mechanically squeezed during this procedure.
2. Intramuscular injection in the home cage. Animals that cooperate during in-home- cage venipunture are readily conditioned to permit intramuscular injection without being mechanically squeezed (Figure 10.3a,b).
3. Removal from home cage. Laboratory rhesus monkeys quickly learn to enter transport cages on command when they have to be temporarily removed for treatment, examination, weighing, TB testing, or breeding, or when they are transferred from their home cage to another cage. No squeeze-backs are necessary to achieve this.

The training of experimental rhesus monkeys is based on positive reinforcement; cooperation is consistently rewarded with favored food such as raisins, peanuts, fruit, or bread (Figure 10.3b). Failure to cooperate is not punished but is never allowed to end a training session, because if it were the animal would quickly learn to avoid any kind of discomfort associated with the handling procedure. Intimate knowledge of the animals, kindness, self-confidence, and patience are key attributes of the caretakers who train the animals at the Wisconsin Regional Primate Research Center. The time investment for the training is minimal (Vertein & Reinhardt, 1989) and quickly pays off in shorter handling times and a more controlled environment for collecting scientific data.


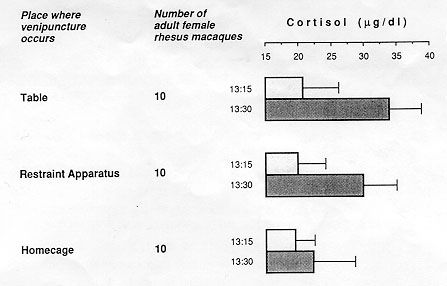
The impact of the training on the quality of data was evaluated for the in-home-cage venipuncture procedure. Significantly elevated peripheral coritsol concentrations were taken as indicators of distress (Clarke, Mason, & Moberg, 1988; Line, Clarke, & Markowitz, 1987; Sassenrath, 1970; Selye, 1971; Tapp, Holaday, & Natelson, 1984; Udelsman & Chrousos, 1988). Cortisol values of 20 singly caged adult female rhesus monkeys routinely subjected to one of two conventional venipuncture techniques (animal is restrained on a table [Figure 10.1] or in a restraint apparatus) were compared with values of 10 singly caged adult female rhesus monkeys subjected to the improved in-home-cage venipunture technique. Each animal was bled by the attending caretaker at 13:15 (assessment of basal cortisol level) and again at 13:30 (assessment of the cortisol response to the preceding venipunture at 13:15). The actual venipuncture occurred for all animals 60 to 90 seconds after the caretaker had entered the animal room. Blood samples were analyzed with a Clinical Assays Gamma Coat Cortisol Kit (Dade, Baxter Travenol Diagnoistics, Cambridge, Mass.)
The three categories of animals did not differ in their mean basal cortisol levels at 13:15 (p always > 0.1; Figure 10.4). At 13:30, however, the mean cortisol concentration of animals bled on the table was significantly higher than that of animals bled in the restraint apparatus (p < 0.1; Figure 10.4) or in the home cage (p < 0.001; Figure 10.4). Being manually immobilized on a table is obviously particularly distressing for rhesus monkeys.
All three categories of test subjects showed a coritsol response to blood collection. The magnitude of cortisol increase, however, was significant only in animals venipunctured on the table ( + 63%, p < .001) or in the restraint apparatus ( + 50%, p < 0.001), but not in animals venipunctured in their home cage ( + 16%, p > 0.1; Figure 10.4). A lack of a significant cortisol response to in-home-cage venipuncture in adult female rhesus monkeys was also reported by Line and collaborators (1987). Apparently, venipunture per se need not be a distressing experience for a rhesus monkey provided that the animal is not removed from its familiar home cage for this procedure.
The present findings indicate that training rhesus monkeys to cooperate during in-home- cage venipuncture is a simple way to increase the validity of research data collected because it helps to avoid undue excitation and associated alteration in basal physiology of the research subjects.
With the refined techniques described here, an experimental monkey can easily be handled by one caretaker, whereas conventional techniques usually require two or three caretakers to control the resisting animal. Once trained, the animals cooperate with any familiar or unfamiliar person who is experienced in working with rhesus monkeys with gentleness and firmness.
The improved handling techniques are based on the experimental subject's cooperation; as such, they are safer for the animal caretaker than the conventional procedures because the monkey no longer has to be forcefully subdued and hence is not unpredictable and ready to defend itself by biting and scratching whenever it can.
Acknowledgments
I am very grateful to Mr. Doug Cowley, Mr. Steve Eisele, and Mr. Russell Vertein for training the animals in their charge to cooperate during routine handling procedures. Thanks are also due to Mrs. Joan Scheffler for analyzing blood samples for cortisol and to Dr. Bill Bridson, Dr. Dan Houser, and Mr. John Wolf for providing valuable comments on this manuscript. The training program is supported by NIH Grant RROO167 to the Wisconsin Regional Primate Research Center.
References
Clarke, A. S., Mason, W., & Moberg, G. P. 1988. Differential behavioral and adrenocortical responses to stress among three macaque species. American Journal of Primatology 14, 37-52.
Line, S. W., Clarke, A. S., & Markowitz, H. 1987. Plasma cortisol of female rhesus monkeys in response to acute restraint. Laboratory Primate Newsletter 26(4), 1-4.
Sassenrath, E. N., 1970. Increased responsiveness related to social stress in rhesus monkeys. Hormones and Behavior 1, 283-98. Selye, H. 1971. Hormones and Resistance.Springer, New York.
Tapp, W. N., Holaday, J. W., & Natelson, B. H. 1984. Ultradian glucocorticoid rhythms in monkeys and rats continue during stress. American Journal of Physiology 247, 866- 871.
Udelsman, R., & Chrousos, G. P. 1988. Hormonal responses to surgical stress. In G. P. Chrousos, D. L. Gold, & P. W. Gold (eds.), Mechanisms of Physical and Emotional Stress, pp. 265-72. Plenum, New York.
Vertein, R., & Reinhardt, V. 1989. Training female rhesus monkeys to cooperate during in-homecage venipuncture. Laboratory Primate Newsletter 28(2), 1-3.
Reprinted with permission of the editor.
This article originally appeared in The Inevitable Bond: Examining Scientist-Animal Interactions, pp. 171-177 / edited by Hank Davis, A. Dianne Balfour. Cambridge University Press 1992.